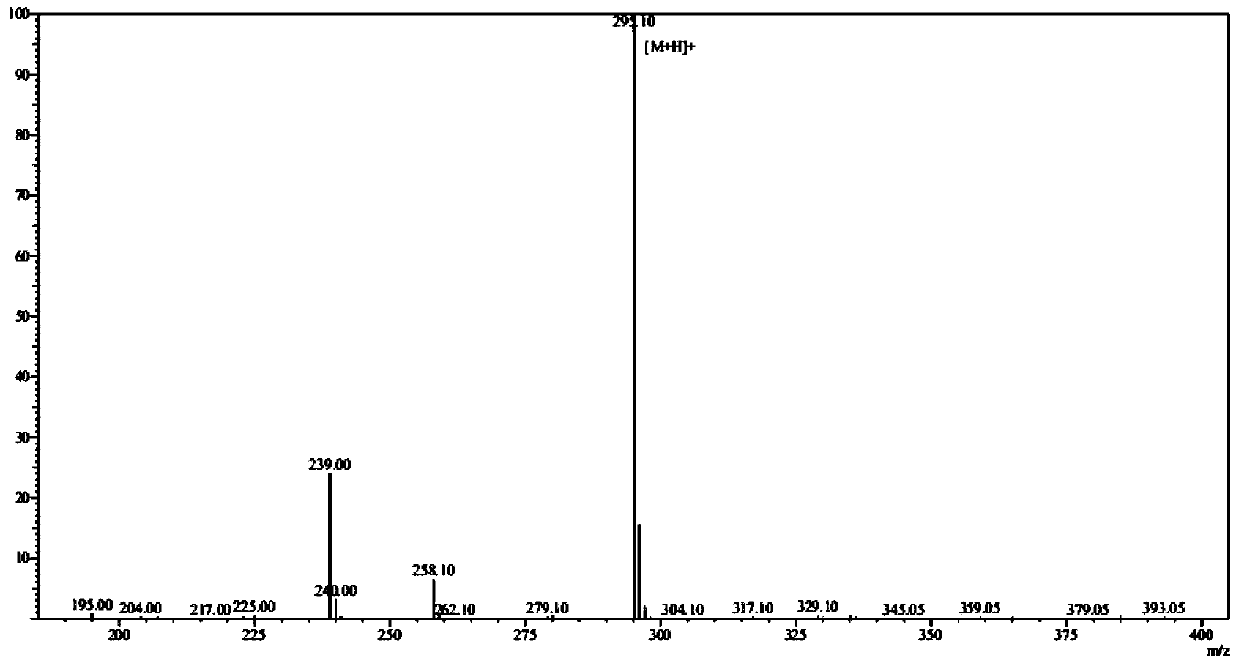
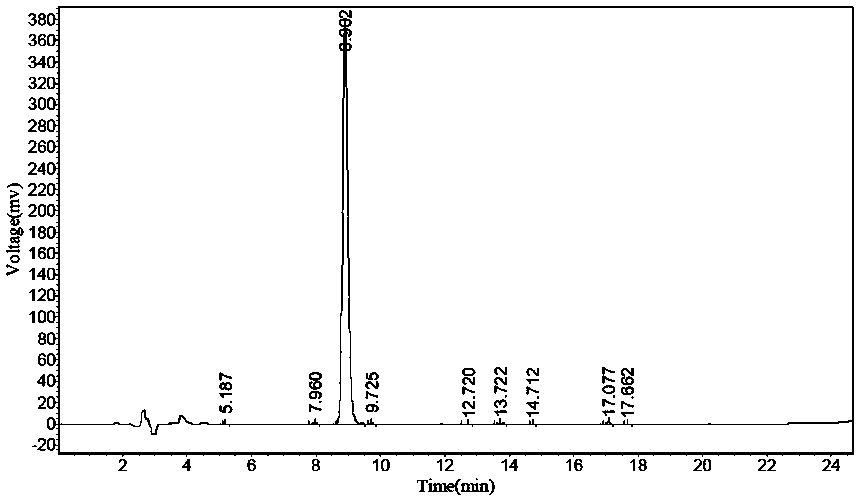
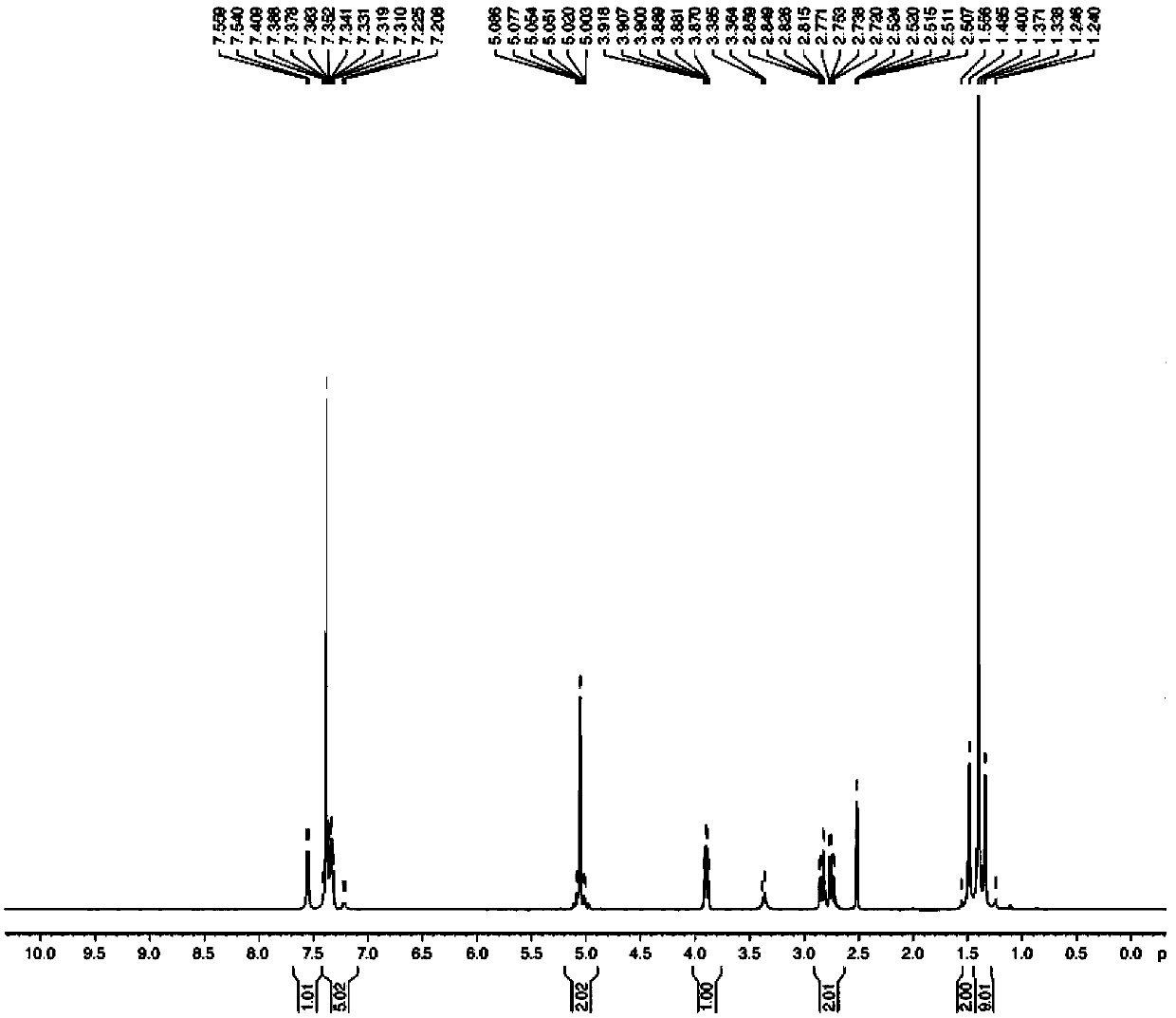
Preparation method of N-carbobenzoxy-3-amino-alanine tert-butyl ester
A technology of tert-butyl alanine and benzyloxycarbonyl, applied in the field of preparation of N-benzyloxycarbonyl-3-amino-alanine tert-butyl ester, can solve the problems of low yield and high cost, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the first step, suspend 10KG asparagine in a mixture of 100KG water and 25KG acetone, stir, adjust the pH value between 8-9 with a 20% mass fraction of sodium carbonate solution, cool down to 0°C, and slowly add chlorine Benzyl formate 16KG, keep the temperature below 3°C, add it in 1 hour, then raise the temperature to 18-20°C to react, and react for 10 hours. According to TLC detection, the reaction of the raw materials was completed, acidified with 4 equivalents of hydrochloric acid to pH = 1, and a solid was precipitated, filtered to obtain the intermediate N-benzyloxycarbonylasparagine, and dried to obtain a solid of 18.14KG, with a yield of 90%.
[0023] In the second step, 18.14KG N-benzyloxycarbonylasparagine is suspended in 330KG dichloromethane, stirred, the temperature is lowered to T<-5°C, 33KG of isobutylene is introduced, and 1.81KG of concentrated sulfuric acid with a mass fraction of 98% is added dropwise. Afterwards, the reaction was heated naturally...
Embodiment 2
[0026] In the first step, suspend 100G asparagine in a mixture of 1000G water and 300G acetone, stir, adjust the pH value between 8-9 with a 20% mass fraction of sodium carbonate solution, cool down to 0°C, and slowly add chlorine Benzyl formate 150G, keep the temperature below 3°C, add in 1-2 hours, then raise the temperature to 18-20°C for reaction, and react for 10 hours. According to TLC detection, the reaction of the raw materials was completed, acidified with 4 equivalents of hydrochloric acid to pH = 1, and a solid was precipitated, filtered to obtain the intermediate N-benzyloxycarbonylasparagine, and dried to obtain a solid of 182G, with a yield of 90.3%.
[0027] In the second step, 182G N-benzyloxycarbonyl asparagine is suspended in 2730G dichloromethane, stirred, the temperature is lowered to T<-5°C, 364G of isobutylene is introduced, and 18.2G of concentrated sulfuric acid with a mass fraction of 98% is added dropwise. Natural heating reaction. Sealed and reacted...
Embodiment 3
[0030]In the first step, suspend 2KG asparagine in a mixture of 20KG water and 4KG acetone, stir, adjust the pH value between 8-9 with a 20% mass fraction of sodium carbonate solution, cool down to 0°C, and slowly add chlorine Benzyl formate is 3.6KG, keep the temperature below 3°C, add it in 2 hours, then raise the temperature to 18-20°C to react, and react for 10 hours. According to TLC detection, the reaction of the raw materials was completed, acidified with 4 equivalents of hydrochloric acid to pH = 1, and a solid was precipitated, and the intermediate N-benzyloxycarbonylasparagine was obtained by filtration, and dried to obtain 3.6KG of a solid, with a yield of 89.3%.
[0031] In the second step, 3.6KG of N-benzyloxycarbonyl asparagine is suspended in 72KG of dichloromethane, stirred, the temperature is lowered to T<-5°C, 5.4KG of isobutylene is introduced, and 0.36KG of concentrated sulfuric acid with a mass fraction of 98% is added dropwise. After completion, the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com