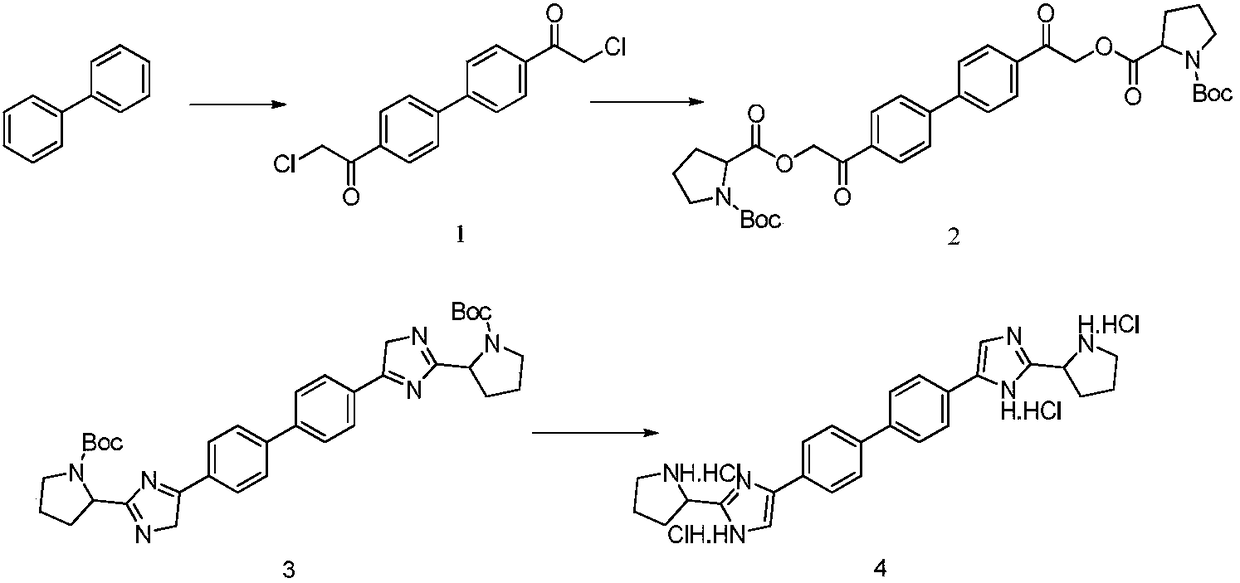
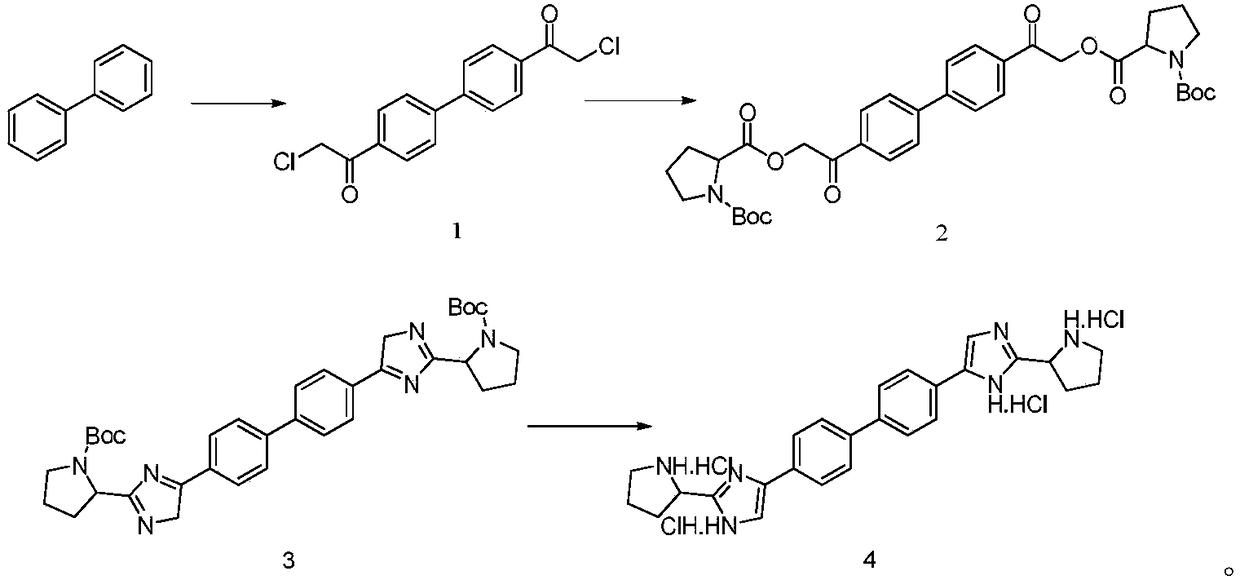
Industrial production method for Daclatasvir key intermediate
A technology of daclatasvir and production method, applied in the field of biochemical industry, can solve the problems of many steps, long synthesis route, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of compound 1
[0027] (1) Under the protection of nitrogen, suck 400kg of methylene chloride into the 1000L reactor, add 80kg of raw material biphenyl (molecular weight 154.2, 519mol) under stirring, stir to dissolve and cool down;
[0028] (2) Cool the reaction system to below 0°C, add 208 kg of anhydrous aluminum chloride (molecular weight 133.3, 1560 mol) in batches, the reaction has obvious exotherm during the addition process, and control the temperature of the reaction system;
[0029] (3) Under the condition of controlling the reaction temperature of the system at 0°C, slowly add 176 kg of chloroacetyl chloride (molecular weight 112.9, 1560 mol) dropwise;
[0030] (4) After the dropwise addition is completed, the reaction system is slowly warmed up to 25°C, and the reaction temperature is maintained, and the liquid phase is controlled until the end of the reaction;
[0031] (5) After the reaction finishes, the reaction solution is directly concentrate...
Embodiment 2
[0048] Synthesis of compound 1
[0049] (1) Under the protection of nitrogen, pump 1000kg of dichloromethane into the 2000L reactor, add 80kg of raw material biphenyl (molecular weight 154.2, 519mol) under stirring, stir to dissolve and cool down;
[0050] (2) Cool the reaction system to below 0°C, add 145 kg of anhydrous aluminum chloride (molecular weight 133.3, 1090 mol) in batches, the reaction has obvious exotherm during the addition process, and control the temperature of the reaction system;
[0051] (3) Under the condition of controlling the reaction temperature of the system at 0°C, slowly add 123 kg of chloroacetyl chloride (molecular weight 112.9, 1090 mol) dropwise;
[0052] (4) After the dropwise addition is completed, the reaction system is slowly warmed up to 25°C, and the reaction temperature is maintained, and the liquid phase is controlled until the end of the reaction;
[0053] (5) After the reaction finishes, the reaction solution is directly concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com