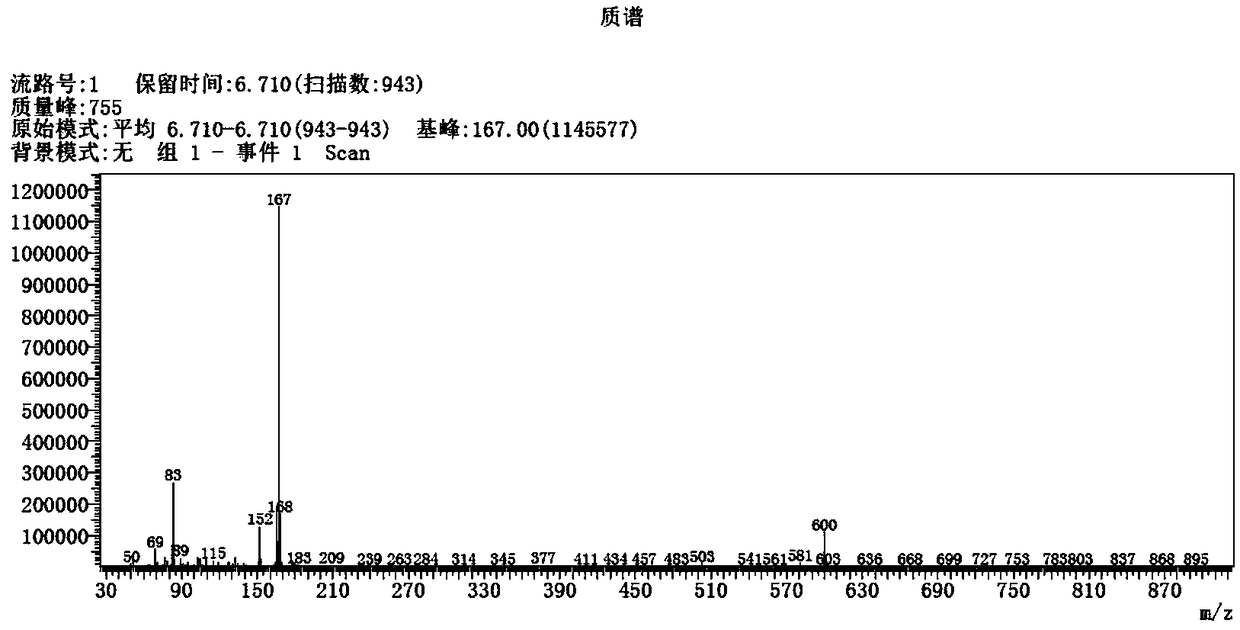
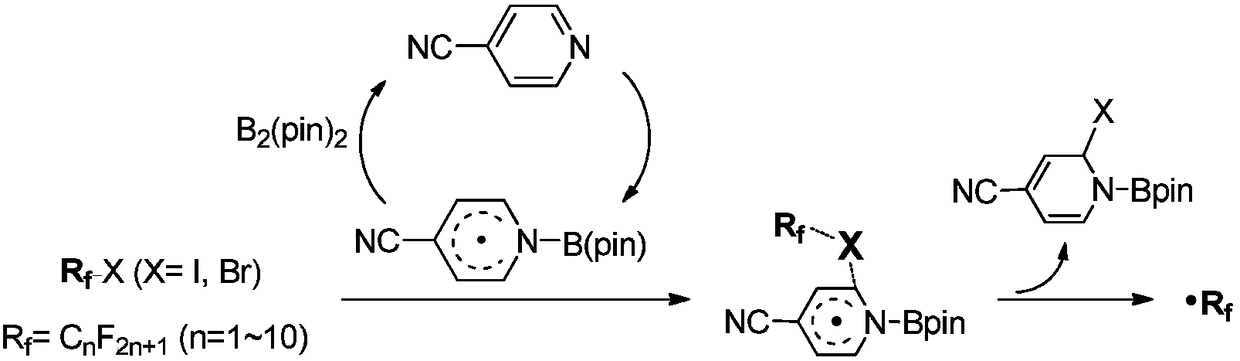
Activation method of perfluoroalkyl carbonhalogen bond and method for synthesizing pyridine derivative
A perfluoroalkyl carbon and perfluoroalkyl technology, applied in the field of organic synthesis, can solve problems such as non-compliance, cumbersome construction of reaction devices, and a large amount of waste liquid, and achieve the effect of solving heavy metal residues and large-scale production in a simple way
Active Publication Date: 2019-03-22
NANJING UNIV
View PDF0 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The traditional methods for introducing pyridine rings include photocatalysis or coupling reaction catalyzed by transition metals. Among them, the photocatalysis method has the advantages of mild conditions and green reaction, but the construction of the reaction device is cumbersome and it is difficult for large-scale industrial production; transition The metal method has the advantages of easy control of the reaction and simple experimental operation, but the catalyst is expensive, heavy metals remain in the product, and a large amount of waste liquid will be generated. The production cost is too high, and it does not meet the requirements of developing modern green chemical industry. Wide range of applications
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0041] 4-(4,4,5,5,6,6,6-heptafluoro-2-(p-methylphenyl)-n-hexyl-2-yl)pyridine, the structural formula is:
[0042]
Embodiment 2
[0044] 4-(4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-2-(p-methylbenzene)n- Undecyl-2-yl)pyridine, the structural formula is:
[0045]
Embodiment 3
[0047] 4-(4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,13-21- 2-(p-toluene)n-tridecane-2-yl)pyridine, the structural formula is:
[0048]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses an activation method of a perfluoroalkyl carbon-halogen bond and a method for synthesizing a pyridine derivative. The activation method is characterized by comprising the stepthat: active pyridine-boron free radicals generated by reaction between 4-cyanopyridine and pinacol biborate are used for catalyzing the homolysis of a carbon-halogen bond of perfluoroalkyl halide toform perfluoroalkyl free radicals. Perfluoroalkyl of olefin and a pyridine difunctionalized pyridine derivatization product are obtained by reaction by taking the pinacol biborate, the perfluoroalkylhalide (or bromide), the 4-cyanopyridine and the olefin as initiators. The method for synthesizing the pyridine derivative, provided by the invention, can simultaneously introduce two important groups, namely the perfluoroalkyl and the pyridine into the olefin for the synthesis of the pyridine derivative, thereby having the value of developing a novel method for the industrial synthesis of a pyridine compound.
Description
technical field [0001] The present application relates to the technical field of organic synthesis, in particular, to a method for activating a perfluoroalkyl carbon-halogen bond and a method for synthesizing pyridine derivatives. Background technique [0002] Fluorine-containing groups can change the physical and chemical properties of substances, and are widely used in medicine, agricultural chemistry and material science, especially in medicine, and can be used as important pharmacophore. Perfluoroalkyl halides are an inexpensive and readily available source of fluorine. However, the activation of perfluoroalkyl carbon-halogen bonds is a key step to achieve the introduction of perfluoroalkyl groups into target compounds. Transition metal catalysis or photocatalysis can effectively activate perfluoroalkyl carbon-halogen bonds, generate perfluoroalkyl carbon radicals, and further realize subsequent transformations. However, these methods usually use transition metals, whi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D213/26C07D405/06C07D401/06C07D213/30C07J43/00
CPCC07D213/26C07D213/30C07D401/06C07D405/06C07J43/003
Inventor 黎书华曹佳王国强高留州
Owner NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com