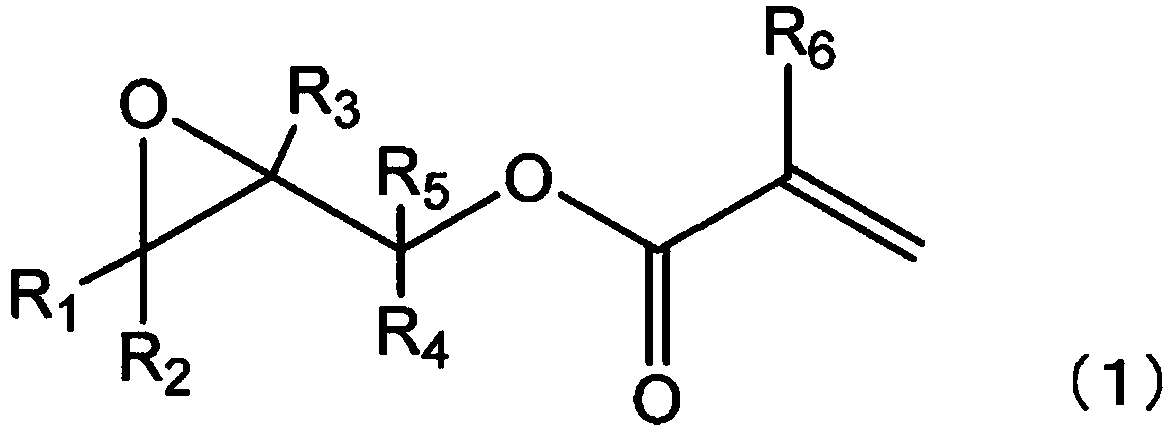
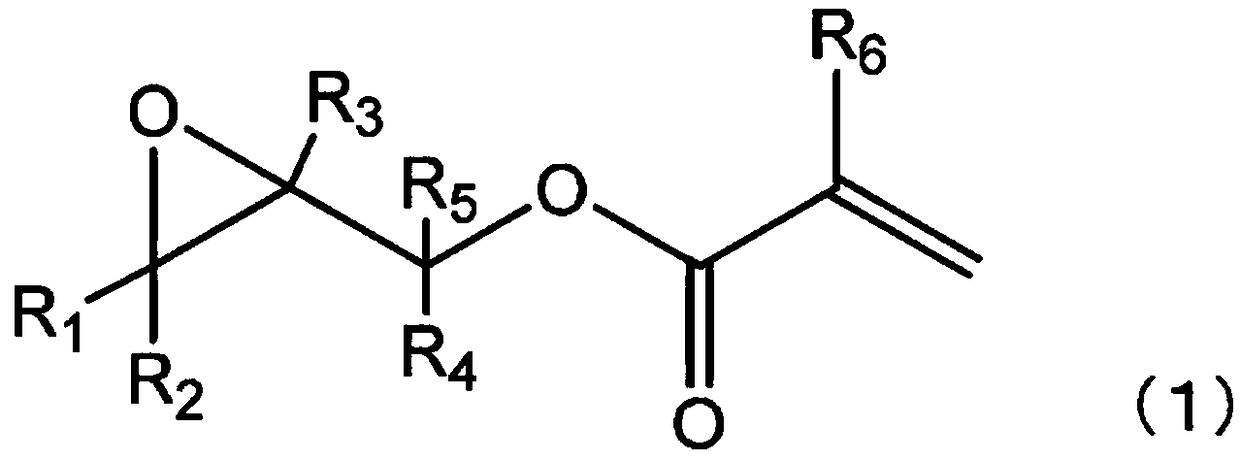
Method for producing ethylenically unsaturated group-containing garma-butyrolactone derivative
A manufacturing method and unsaturated technology, applied in the fields of organic chemistry, organic chemistry, etc., can solve the problems of difficult compound manufacturing, poor reaction selectivity, and difficult to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
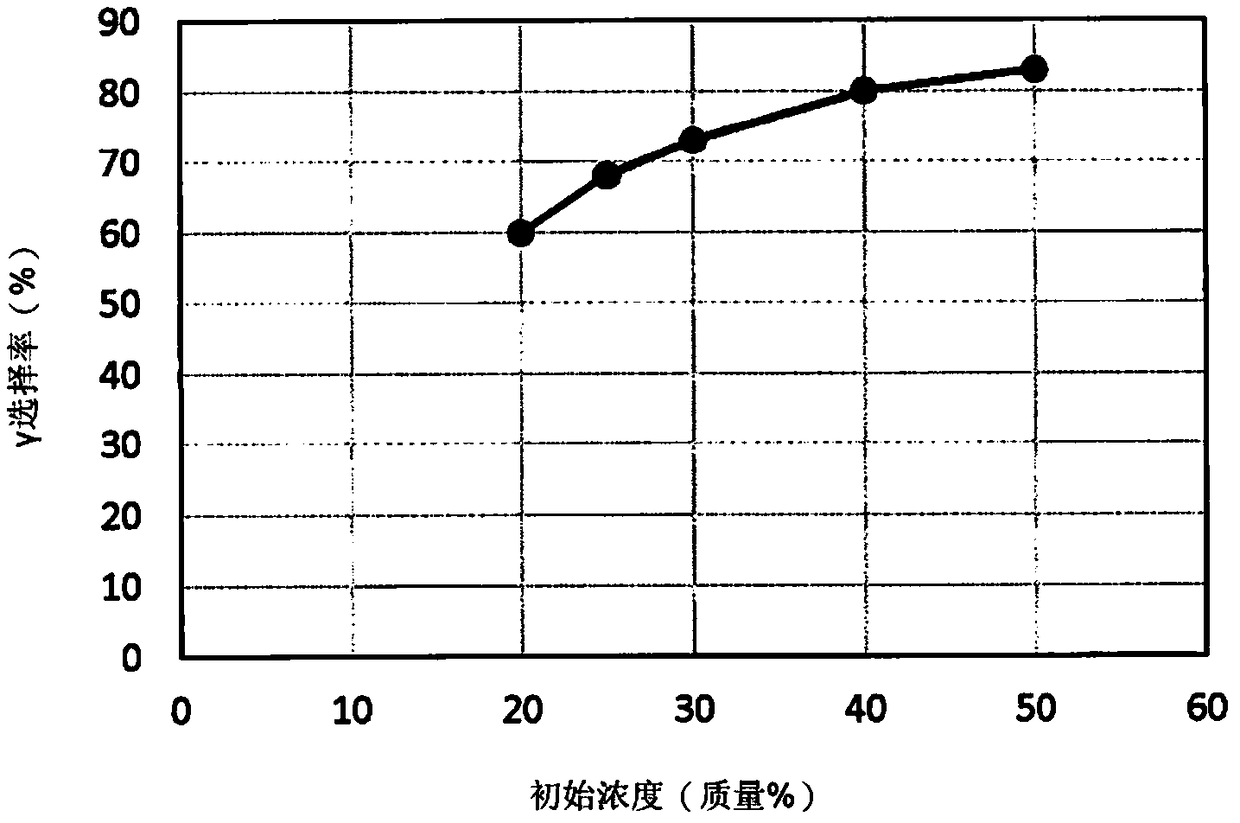
[0129] In a glove box under a nitrogen atmosphere, 21.3 g of glycidylmethacrylate (Glycidylmethacrylate, GMA), 85.24 g of 2-acetoxy-1-methoxypropane, 0.02 g of 4,4′-methylene Bis(2,6-di-tert-butylphenol) (BBHT), 3.0 ml of 1 mol / L triethylaluminum (15% hexane solution, manufactured by Wako Pure Chemical Industries), 0.256 g of dicobalt octacarbonyl into a 200 ml autoclave container and mixed (initial concentration: 20% by mass). Install the CO gas line into the autoclave, and perform three replacements with CO gas. CO gas was introduced until the pressure became 5.0 MPa and heated. The reaction was carried out for 240 minutes at a temperature of 90° C. and an internal pressure of 4.8 MPa to 5.0 MPa.
[0130] After the reaction finishes, utilize GC (GC device; Shimadzu Corporation GC-2014, column; Agilent J&W (AgilentJ&W) capillary GC column DB-1 60m * 0.25mmID * 0.25μm) to glycidyl methacrylate Quantitative analysis of β-methacryloyloxymethyl-β-propiolactone and β-methacrylo...
experiment example 2
[0133] The reaction was carried out in the same manner as in Experimental Example 1 except that 4,4'-methylenebis(2,6-di-tert-butylphenol) was not added.
[0134] After the reaction, use GC to quantitatively analyze glycidyl methacrylate, and use HPLC to analyze β-methacryloyloxymethyl-β-propiolactone and β-methacryloyloxy-γ-butylene Quantitative analysis of lactones. As a result, the conversion rate of glycidyl methacrylate was 99.4%, the lactone production rate was 62.7%, and β-methacryloyloxy-γ-butyrolactone (γ) The ratio of methyl-β-propiolactone (β) is γ:β=62:38. Table 1 shows the experimental conditions and results.
experiment example 3
[0136] In a glove box under a nitrogen atmosphere, 27.0 g of glycidyl methacrylate, 76.0 g of 2-acetoxy-1-methoxypropane, 0.03 g of 4,4'-methylenebis(2, 6-di-tert-butylphenol), 3.8ml of 1mol / L triethylaluminum (15% hexane solution, manufactured by Wako Pure Chemical Industries), 0.325g of dicobalt octacarbonyl were placed in a 200ml autoclave container and Mixing (initial concentration: 25% by mass). Install the CO gas line into the autoclave, and perform three replacements with CO gas. CO gas was introduced until the pressure became 5.0 MPa and heated. The reaction was carried out for 240 minutes at a temperature of 90° C. and an internal pressure of 4.8 MPa to 5.0 MPa.
[0137] After the reaction, use GC to quantitatively analyze glycidyl methacrylate, and use HPLC to analyze β-methacryloyloxymethyl-β-propiolactone and β-methacryloyloxy-γ-butylene Quantitative analysis of lactones. As a result, the conversion rate of glycidyl methacrylate was 99.4%, and the lactone produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com