Intratumoral administration of particles containing a toll-like receptor 9 agonist and a tumor antigen for treating cancer
A technology of tumor antigens and agonists, applied in medical preparations containing active ingredients, vertebrate antigen components, carrier-antigen complex structures, etc., can solve the problems of not improving the survival rate of patients with non-small cell lung cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
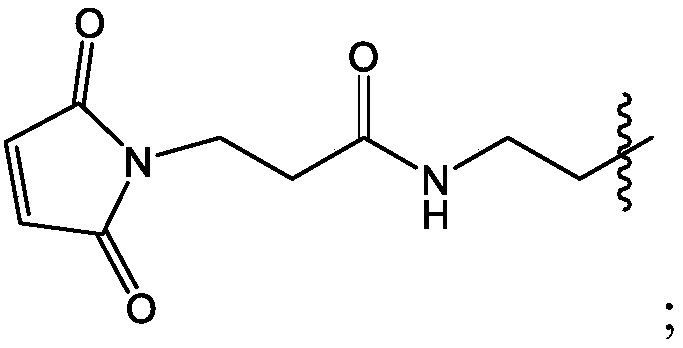
[0286] Abbreviations: Ab (antibody); Ag (antigen); Alum (aluminum salt adjuvants, such as those sold by Brenntag Nordic A / S 85); Al(OH) 3 (aluminum hydroxide); AlPO 4 (aluminum phosphate); BMDC (bone marrow-derived dendritic cells); CC (chimeric compound); CpG (polynucleotide comprising unmethylated CG dinucleotide or its chimeric compound); CTAG1 (cancer / testis antigen 1); CTRL (control); DC (dendritic cells); ELISA (enzyme-linked immunosorbent assay); EC 50 (half maximum effective concentration); FACS (fluorescence activated cell sorting); FCS (fetal calf serum); Fic (branched copolymer of high molecular weight sucrose and epichlorohydrin, such as FICOLL sold by GE Medical); HEG ( Hexaethylene glycol); HLA (human leukocyte antigen); Hypb (hydrophobic); IFN-γ (interferon-γ); IPA (isopropanol); IT (intratumoral); mcg or μg (micrograms); MAGEA ( Melanoma antigen, family A); MW (molecular weight); MWCO (molecular weight cut-off); NaCl (sodium chloride); NaOAc (sodium acetat...
Embodiment S1
[0288] Example S1: Structures of polynucleotides and chimeric compounds
[0289] Table S1-1 shows the structures of polynucleotides and chimeric compounds, which are generally referred to herein interchangeably as CpG or CpG-ODN. The nucleotides in polynucleotides and chimeric compounds are 2'-deoxyribose polynucleotides. HEG is a hexaethylene glycol spacer part and uses 18-O-dimethoxytrityl hexaethylene glycol, 1-((2-cyanoethyl)-(N,N-isopropyl))- Phosphoramide incorporated. All internucleotide linkages and linkages between nucleic acid moieties and spacer moieties are phosphorothioate linkages.
[0290] Table S1-1: Polynucleotide (PN) and chimeric compound (CC) structures^
[0291]
[0292] ^ Unless otherwise stated, the polynucleotides and chimeric compounds of SEQ ID NO: 5, 6, 7, 8, 9, 10 and 27 include 2'-deoxy-ribose polynucleotides and the internucleotide linkages are Phosphorothioate linkage. Different compounds are given the same SEQ ID NO when the only differe...
Embodiment S2
[0294] Example S2: Structure of polypeptide antigen
[0295] Table S2-1 shows the primary structures of polypeptide antigens, which are interchangeably referred to herein as polypeptides or peptides. Polypeptides were purchased from Bio-Synthesis Inc. (Lewisville, Texas, USA) or C S Bio (Menlo Park, California, USA).
[0296] Table S2-1: Peptide structure
[0297]
[0298]
[0299] ^The hydrophobicity (Hypb) was determined using the online peptide property calculator found at "www.biosyn.com / peptidepropertycalculatorlanding.aspx" and expressed as a percentage of the full-length amino acid sequence. (polyethylene glycol) 24 / PEG 24 and Cyclohexylalanine are not included in the Hypb calculation.
[0300] The OVApep antigen includes the ovalbumin (OVA) class I epitope along with seven N-terminal amino acids from OVA to facilitate peptide excision (Cascio et al., 2001 EMBO J, 20:2357–2366) and the OVA class II epitope (Maecker et al., 1998 J Immunol, 161:6532-6536). A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com