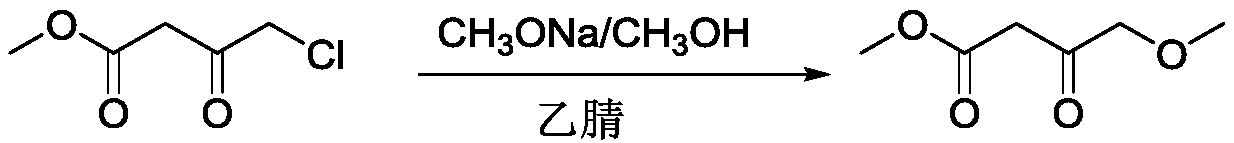
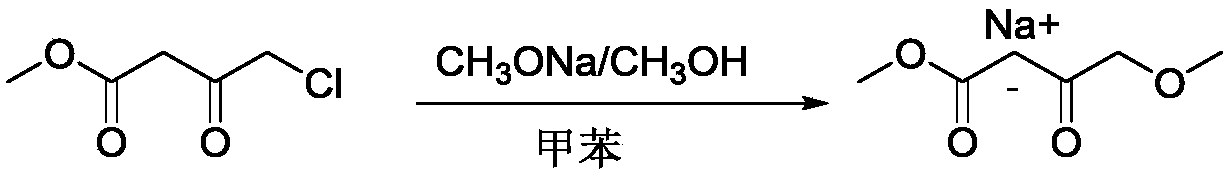
Preparation process of methyl 4-methoxyacetoacetate
A technology for the preparation of methyl methoxyacetoacetate, which is applied in the field of medicinal chemistry, can solve the problems of high risk and high cost, and achieve the effects of safe and reliable production, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Under nitrogen protection, in a 5L four-necked flask, add 1500ml of anhydrous toluene, 400g (2.2mol) of 30% sodium methoxide in methanol, and dropwise add 150.6g (1.0 mol) of 4-chloroacetoacetate methyl ester at 20-25°C mol), after the dropwise addition, continue to stir at room temperature for 2 hours; heat to 65-70°C, keep warm for 3h, cool to room temperature, and produce a large amount of white solid. Adjust the pH to 5-6 with 2N hydrochloric acid, and continue stirring for 30 minutes. Adjust the pH to 3-4 with 2N hydrochloric acid, separate the toluene layer; extract the water layer once with 500ml of toluene. The toluene layers were combined and washed with saturated sodium chloride solution until neutral. Distill under reduced pressure to remove toluene to obtain crude product. The crude product was distilled under reduced pressure (90-100°C / 10mmHg) to obtain 108 g of a colorless transparent liquid with a GC purity of 99% and a yield of 74%.
[0025] Product H...
Embodiment 2
[0028] Under nitrogen protection, in a 5L four-necked flask, add 1500ml of anhydrous toluene, 185g (1.0mol) of 30% sodium methoxide in methanol, and dropwise add 150.6g (1.0mol) of 4-chloroacetoacetate methyl ester at 20-25°C. mol), after the dropwise addition, continue to stir at room temperature for 2 hours; heat to 65-70°C, keep warm for 3h, cool to room temperature, and produce a large amount of white solid. Adjust the pH to 6 with acetic acid, and continue stirring for 30 minutes. Adjust the pH to 3-4 with 2N hydrochloric acid, separate the toluene layer; extract the water layer once with 500ml of toluene. The toluene layers were combined and washed with saturated sodium chloride solution until neutral. Distill under reduced pressure to remove toluene to obtain crude product. The crude product was distilled under reduced pressure (90-100°C / 10mmHg) to obtain 58 g of a colorless transparent liquid with a GC purity of 99% and a yield of 40%.
[0029] Product H Spectrum: ...
Embodiment 3
[0032] Under nitrogen protection, in a 5L four-necked flask, add 1500ml of anhydrous toluene, 400g (2.2mol) of 30% sodium methoxide in methanol, and dropwise add 150.6g (1.0 mol) of 4-chloroacetoacetate methyl ester at 20-25°C mol), after the dropwise addition, continue to stir at room temperature for 2 hours; heat to 65-70°C, keep warm for 3h, cool to room temperature, and produce a large amount of white solid. Adjust the pH to 6 with acetic acid, and continue stirring for 30 minutes. Adjust the pH to 3-4 with 2N hydrochloric acid, separate the toluene layer; extract the water layer once with 500ml of toluene. The toluene layers were combined and washed with saturated sodium chloride solution until neutral. Distill under reduced pressure to remove toluene to obtain crude product. The crude product was distilled under reduced pressure (90-100°C / 10mmHg) to obtain 110 g of a colorless transparent liquid with a GC purity of 99% and a yield of 75%.
[0033] Product H Spectrum: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com