Comprehensive recovery method for waste lithium ion battery
A technology for lithium-ion batteries and waste lithium batteries, applied in battery recycling, recycling by waste collectors, recycling technology, etc., can solve problems such as inability to achieve high-efficiency and priority extraction of lithium, and achieve harmless disposal and high-efficiency priority extraction of lithium , the effect of effective recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
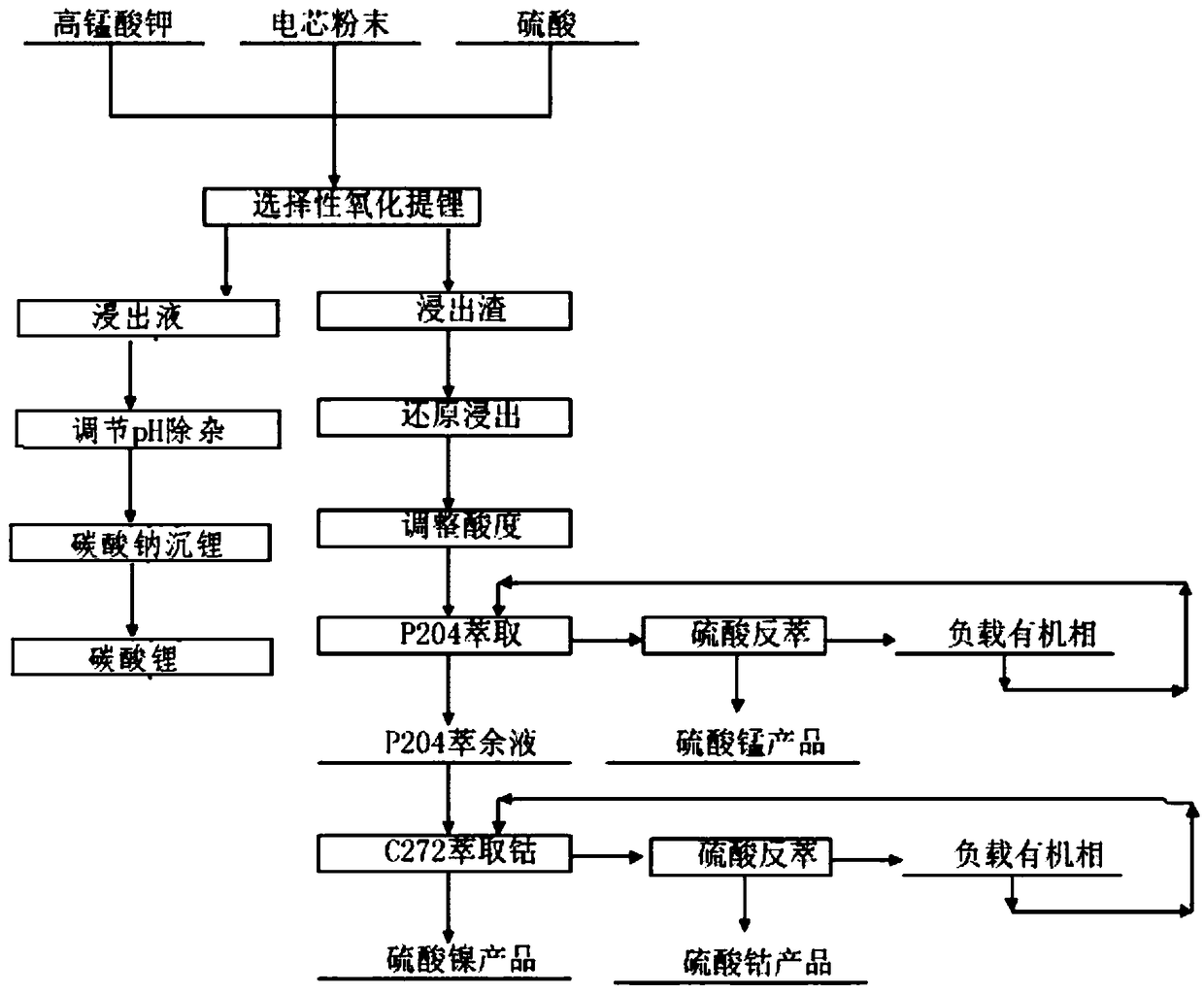
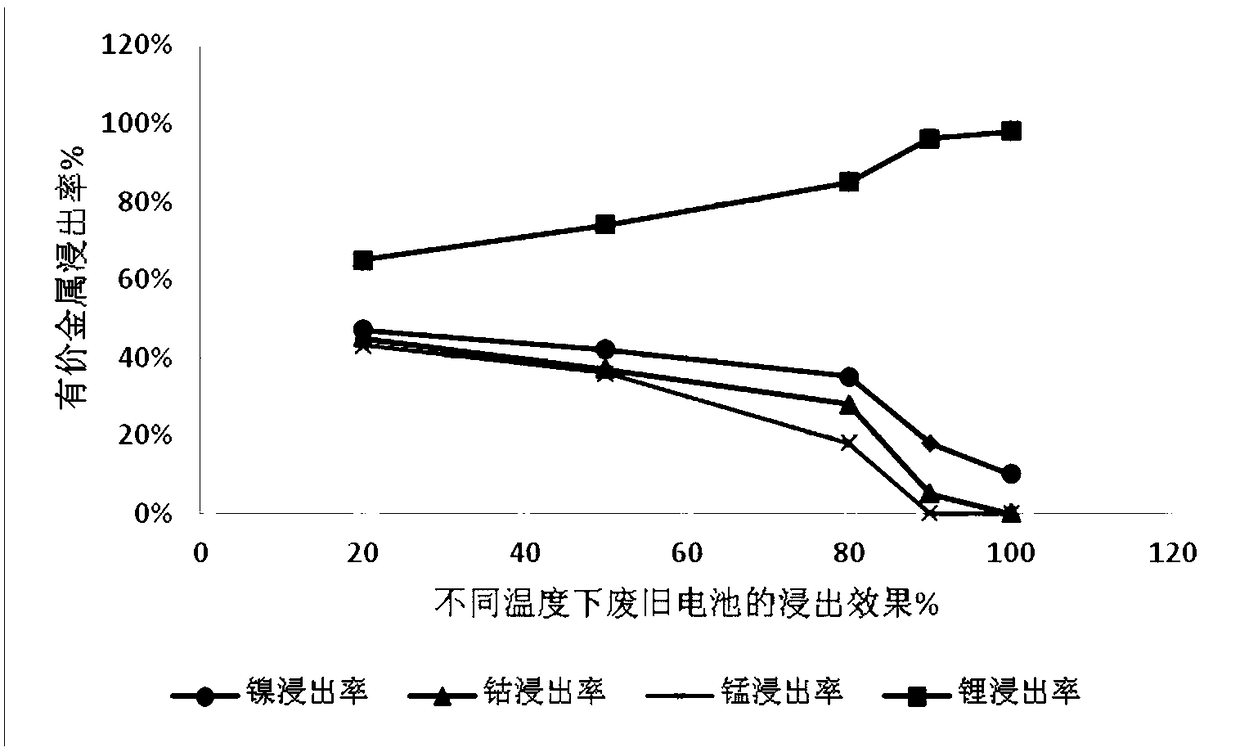
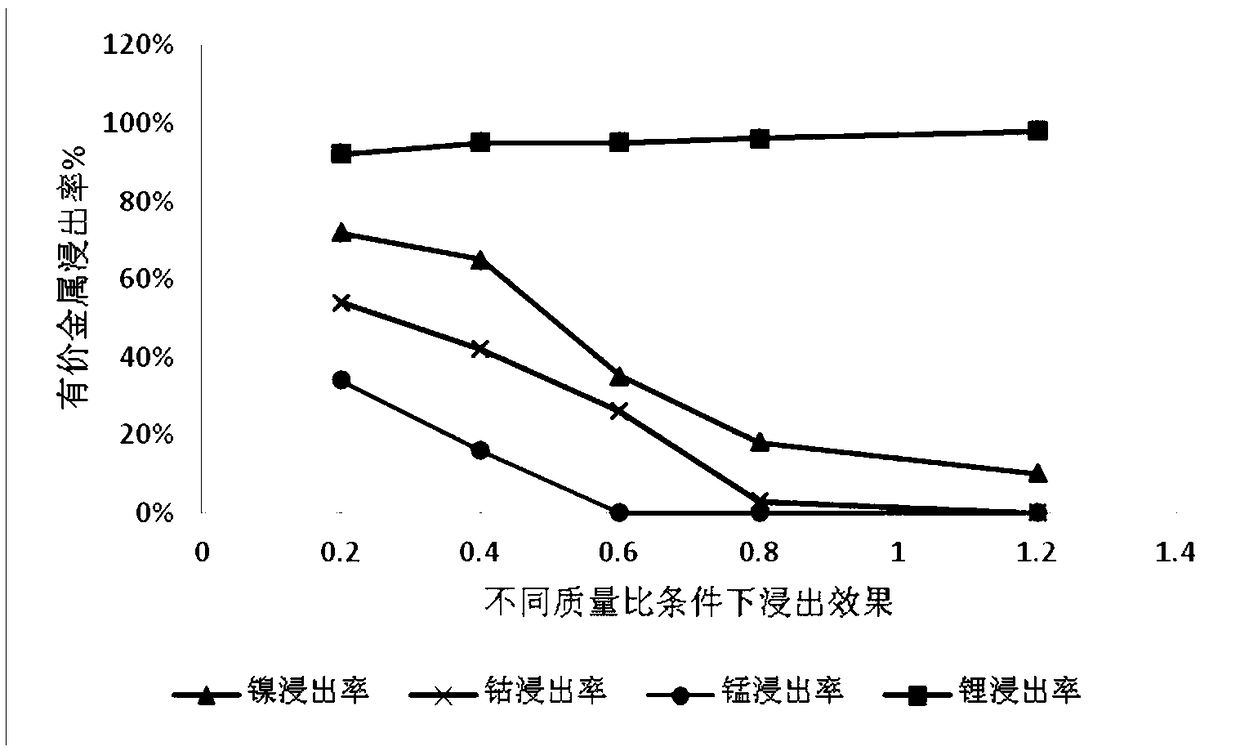
[0035] Get ternary waste lithium battery powder 50g, add 4mol / L sulfuric acid, add 10g additive potassium permanganate (KMnO 4 ), at 20°C, stirred and reacted for 2 hours, then filtered to obtain a mixed solution containing nickel sulfate, cobalt sulfate, manganese sulfate and lithium sulfate and leaching residue in the filtrate. The leaching effect is as follows: figure 1 , 2 and 3. Adjust the pH of the filtrate to ≈5.5, remove impurities, and obtain a high-concentration lithium-containing solution. The lithium-containing solution is subjected to a lithium precipitation process using sodium carbonate to obtain a pure lithium carbonate product.
[0036]The leaching residue is reduced and leached with sulfuric acid and hydrogen peroxide, the sulfuric acid is 1.1 times the theoretical amount, and the hydrogen peroxide is 1.4 times the theoretical amount. Under the condition of 60°C, a high-concentration mixed solution containing nickel sulfate, cobalt sulfate and manganese sulf...
Embodiment 2
[0040] Take 50g of ternary waste lithium battery cell powder, add 4mol / L sulfuric acid, add 30g of additive KMnO 4 , under the condition of 50 ℃, after stirring and reacting for 4 hours, filter to obtain a solution containing nickel-cobalt-lithium and leaching residue, the leaching effect is shown in figure 1 , 2 And 3, adjust the filtrate pH ≈ 5-5.5, remove impurities, obtain a high-concentration lithium-containing solution, and then use sodium carbonate to perform a lithium-precipitation process on the lithium-containing solution to obtain a lithium carbonate product.
[0041] The leaching residue is reduced and leached with sulfuric acid and hydrogen peroxide. The sulfuric acid is 1.05 times the theoretical amount, and the hydrogen peroxide is 1.3 times the theoretical amount. At 60°C, a high-concentration lithium-containing solution is obtained, wherein nickel: 61.75g / L, cobalt: 23.75g / L, manganese: 35.15g / L. Adjust the pH of the lithium-containing solution to ≈4.5, and...
Embodiment 3
[0045] Take 50g of ternary waste lithium battery cell powder, add 4mol / L sulfuric acid, add additive KMnO 4 40g, under the condition of 80 ℃, after stirring and reacting for 12h, filter to obtain a solution containing nickel lithium manganese (leaching effect see figure 1 , 2 , 3), adjust the filtrate pH ≈ 5-5.5, remove impurities, obtain a high-concentration lithium-containing solution, and then perform a lithium precipitation process to obtain a lithium carbonate product
[0046] The leaching residue is reduced and leached with sulfuric acid and hydrogen peroxide. The sulfuric acid is 0.95 times of the theoretical amount, and the hydrogen peroxide is 1.1 times of the theoretical amount. At 60°C, a high-concentration nickel-cobalt-manganese-containing solution is obtained, nickel: 55.25g / L, cobalt: 21.25g / L, manganese: 31.45g / L, adjust the pH of the solution to ≈4.5, and use 25% P204 to extract and remove impurities after nickel soap. The extraction stage is 12 to remove co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com