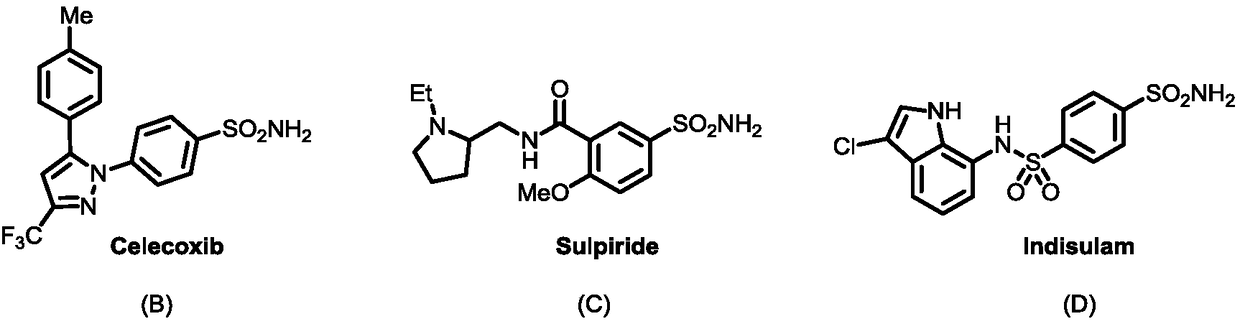
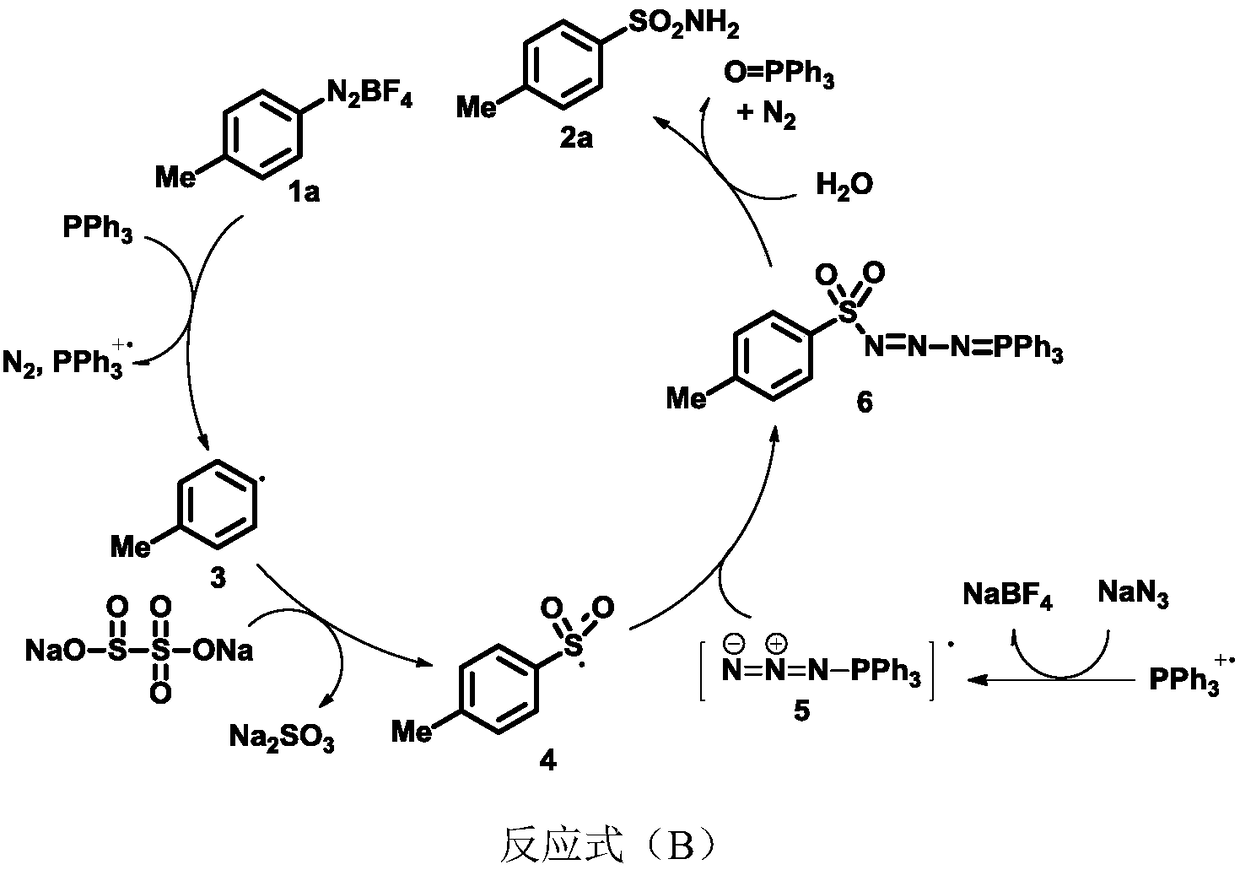
Sulfonamide compound and synthesis method and application thereof
A synthetic method and technology of sulfonamides, applied in the field of organic compound synthesis and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Compound 2a:
[0044]
[0045] Under nitrogen protection, the diazonium salt 1a (257.4mg, 1,25mmol), NaN 3 (32.5mg, 0.5mmol), PPh 3 (157.4mg, 0.6mmol), Na 2 S 2 o 5 (190.1mg, 1.0mmol), TBAB (241.7mg, 0.75mmol) and MeCN / H 2 O=2 / 1 (1 mL) was added to the Schlenk reaction tube. After the reaction was stirred at 80°C for 12 hours, it was lowered to room temperature, and 10 mL of water was added to the system for dilution, then ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a white solid 2a (90%). 1 H NMR (400MHz, d 6 -DMSO) δ7.71(d, J=8.2Hz, 2H), 7.36(d, J=8.1Hz, 2H), 7.27(s, 2H), 2.37(s, 3H); 13 C NMR (100MHz, d 6 -DMSO) δ141.8, 141.4, 129.3, 125.6, 20.9; IR (KBr) ν3348, 3270, 1598, 1500, 1309, 1175, 1021, 917, 835, 669cm -1 ; HRMS (EI) for C 7 h 9 NO 2 SCalculated: 171.0354,found: 171.0353.
Embodiment 2
[0047] Synthesis of compound 2b:
[0048]
[0049] Under nitrogen protection, the diazonium salt 1b (239.9mg, 1.25mmol), NaN 3 (32.5mg, 0.5mmol), PPh 3 (157.4mg, 0.6mmol), Na 2 S 2 o 5 (190.1mg, 1.0mmol), TBAB (241.7mg, 0.75mmol) and MeCN / H 2 O=2 / 1 (1 mL) was added to the Schlenk reaction tube. After the reaction was stirred at 80°C for 12 hours, it was lowered to room temperature, and 10 mL of water was added to the system for dilution, then ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a white solid 2b (14.8 mg, 72%). 1 H NMR (400MHz, d 6 -acetone) δ7.91 (d, J=7.0Hz, 2H), 7.72–7.43 (m, 3H), 6.59 (s, 2H); 13 C NMR (100MHz, d 6 -acetone)δ145.0, 132.8, 129.7, 126.8; IR(KBr)ν3353, 3258, 1448, 1344, 1160, 1091, 756, 688, 537cm -1 ; HRMS (EI) for C 6 h 7 NO 2 S Calculated: 157.0197, Found: 157.0200.
Embodiment 3
[0051] Synthesis of compound 2c:
[0052]
[0053] Under nitrogen protection, the diazonium salt 1c (277.4mg, 1.25mmol), NaN 3 (32.5mg, 0.5mmol), PPh 3 (157.4mg, 0.6mmol), Na 2 S 2 o 5 (190.1mg, 1.0mmol), TBAB (241.7mg, 0.75mmol) and MeCN / H 2 O=2 / 1 (1 mL) was added to the Schlenk reaction tube. After the reaction was stirred at 80°C for 12 hours, it was lowered to room temperature, and 10 mL of water was added to the system for dilution, then ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a white solid 2c (91%). 1 H NMR (400MHz, d 6 -acetone) δ7.83(d, J=8.9Hz, 2H), 7.06(d, J=8.9Hz, 2H), 6.43(s, 2H), 3.87(s, 3H); 13 C NMR (100MHz, d 6 -acetone)δ163.2,137.0,128.9,114.7,56.0; IR(KBr)ν3348,3270,2980,1598,1500,1309,1157,917,835,669cm -1 ; HRMS (EI) for C 7 h 9 NO 3 SCalculated: 187.0303,found: 187.0306.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com