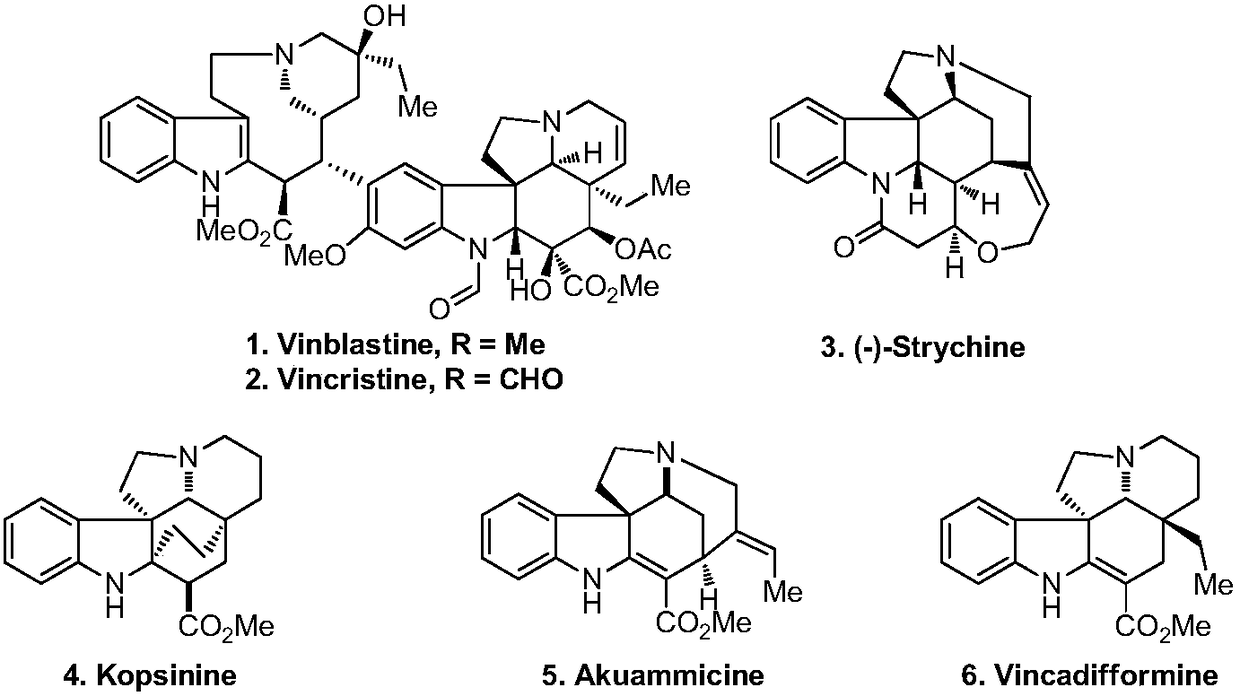
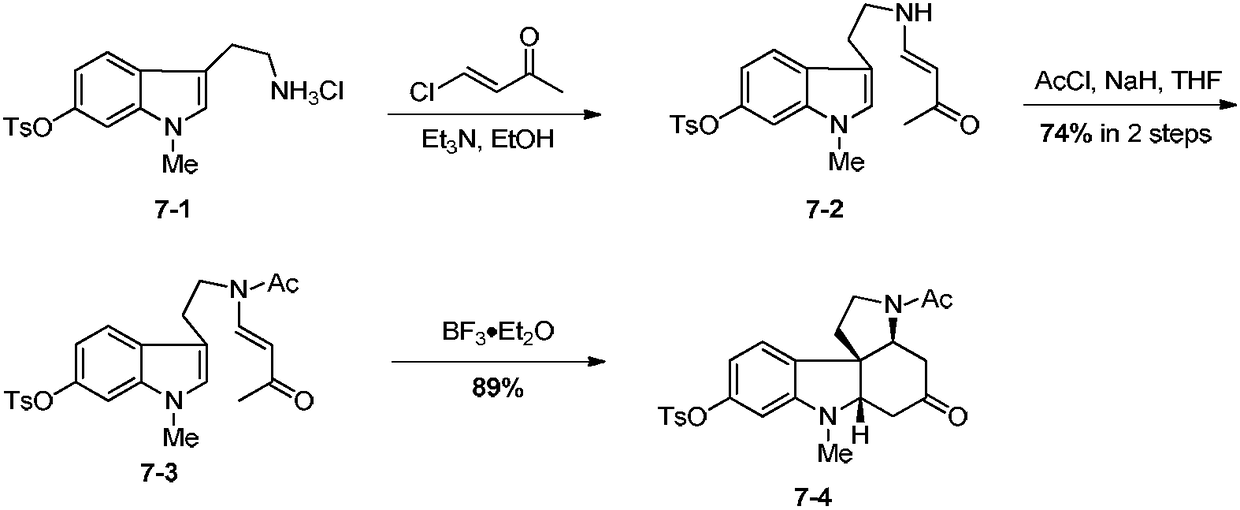
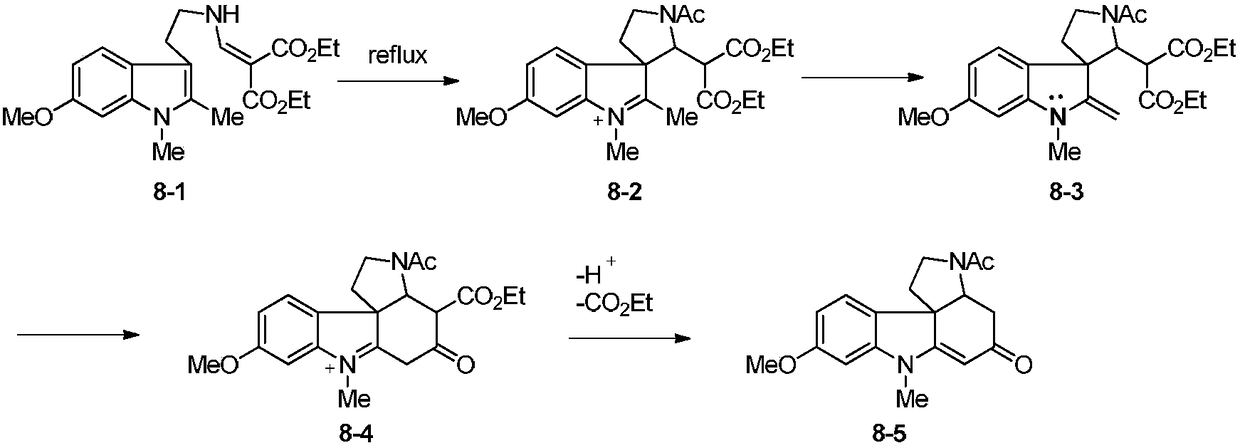
Synthetic method for tetracyclic indole skeletons under catalysis of protonic acid
A tetracyclic indole and synthetic method technology, applied in the direction of organic chemistry, can solve the problems of cumbersome routes, harsh experimental conditions, low yield, etc., and achieve the effect of simple preparation of substrates and simple and convenient preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Substrate 1-1 (1 mmol) was dissolved in dry dichloromethane (10 mL), and diphenyl phosphate (0.1 mmol, 25 mg) was added under ice-cooling, and reacted under ice-bath for 1 h. After the reaction is completed, use petroleum ether / ethyl acetate (v / v=6 / 1) as the eluent system, and use 200-300 mesh silica gel as the stationary phase to perform column chromatography separation to finally obtain the target compound 1-2. The yield was 82%. Its reaction equation is:
[0046]
[0047]The spectral data of product 1-2 is: HRMS (ESI) m / z: [M+H] + 501.1848; 1 H NMR (600MHz, DMSO-d 6 )δ=7.94(d, J=8.2Hz, 2H), 7.55(d, J=8.2Hz, 2H), 7.39(d, J=7.3Hz, 2H), 7.34(t, J=7.4Hz, 2H) ,7.26(t,J=7.4Hz,1H),6.60(dd,J=8.5,2.5Hz,1H),6.33(d,J=8.5Hz,1H),5.99(s,1H),5.39(d, J=2.5Hz, 1H), 4.24(d, J=15.1Hz, 1H), 4.10–4.06(m, 2H), 3.95(dd, J=9.9, 5.9Hz, 1H), 3.73(td, J=10.7 ,5.6Hz,1H),3.47(s,3H),2.46–2.41(m,4H),2.20(td,J=11.8,8.4Hz,1H),2.01–1.99(m,1H),1.89(dd, J = 16.7, 10.0 Hz, 1H).
Embodiment 2
[0049] Substrate 2-1 (1 mmol) was dissolved in dry dichloromethane (10 mL), and diphenyl phosphate (0.1 mmol, 25 mg) was added under ice-cooling, and reacted under ice-bath for 1 h. After the reaction is completed, use petroleum ether / ethyl acetate (v / v=6 / 1) as the eluent system, and use 200-300 mesh silica gel as the stationary phase to perform column chromatography separation to finally obtain the target compound 2-2. The yield was 72%. Its reaction equation is:
[0050]
[0051] The spectral data of product 2-2 is: HRMS (ESI) m / z: [M+Na] + 527.1151; 1 H NMR (600MHz, DMSO-d 6 )δ=7.95(d,J=8.2Hz,2H),7.58(d,J=8.2Hz,2H),7.38–7.33(m,4H),7.29–7.25(m,1H),7.02(dd,J =8.4,2.1Hz,1H),6.41(d,J=8.4Hz,1H),6.06(s,1H),5.42(d,J=2.1Hz,1H),4.32(d,J=15.3Hz,1H ),4.17(d,J=15.3Hz,1H),4.09–4.01(m,2H),3.67(td,J=10.5,5.6Hz,1H),2.53(d,J=6.0Hz,1H),2.45 (s,3H),2.22(td,J=11.8,8.3Hz,1H),2.03–1.97(m,1H),1.90(dd,J=16.8,9.9Hz,1H)
Embodiment 3
[0053] Substrate 3-1 (1 mmol) was dissolved in dry dichloromethane (10 mL), and diphenyl phosphate (0.1 mmol, 25 mg) was added under ice-cooling, and reacted under ice-bath for 1 h. After the reaction is completed, use petroleum ether / ethyl acetate (v / v=6 / 1) as the eluent system, and use 200-300 mesh silica gel as the stationary phase to perform column chromatography separation to finally obtain the target compound 3-2. Reaction The yield is 75%. Its reaction equation is:
[0054]
[0055] The spectral data of product 3-2 is: HRMS (ESI) m / z: [M+Na] + 571.0638; 1 H NMR (600MHz, DMSO-d 6 )δ=7.95(d, J=8.2Hz, 2H), 7.58(d, J=8.2Hz, 2H), 7.37–7.33(m, 4H), 7.27(t, J=6.7Hz, 1H), 7.15( dd,J=8.4,1.9Hz,1H),6.38(d,J=8.4Hz,1H),6.05(s,1H),5.62(d,J=1.8Hz,1H),4.32(d,J=15.3 Hz, 1H), 4.17(d, J=15.3Hz, 1H), 4.11–4.00(m, 2H), 3.67(td, J=10.7, 5.5Hz, 1H), 2.53(d, J=6.0Hz, 1H ),2.46(s,3H),2.23(dt,J=11.7,8.4Hz,1H),2.06–1.97(m,1H),1.90(dd,J=16.8,9.9Hz,1H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com