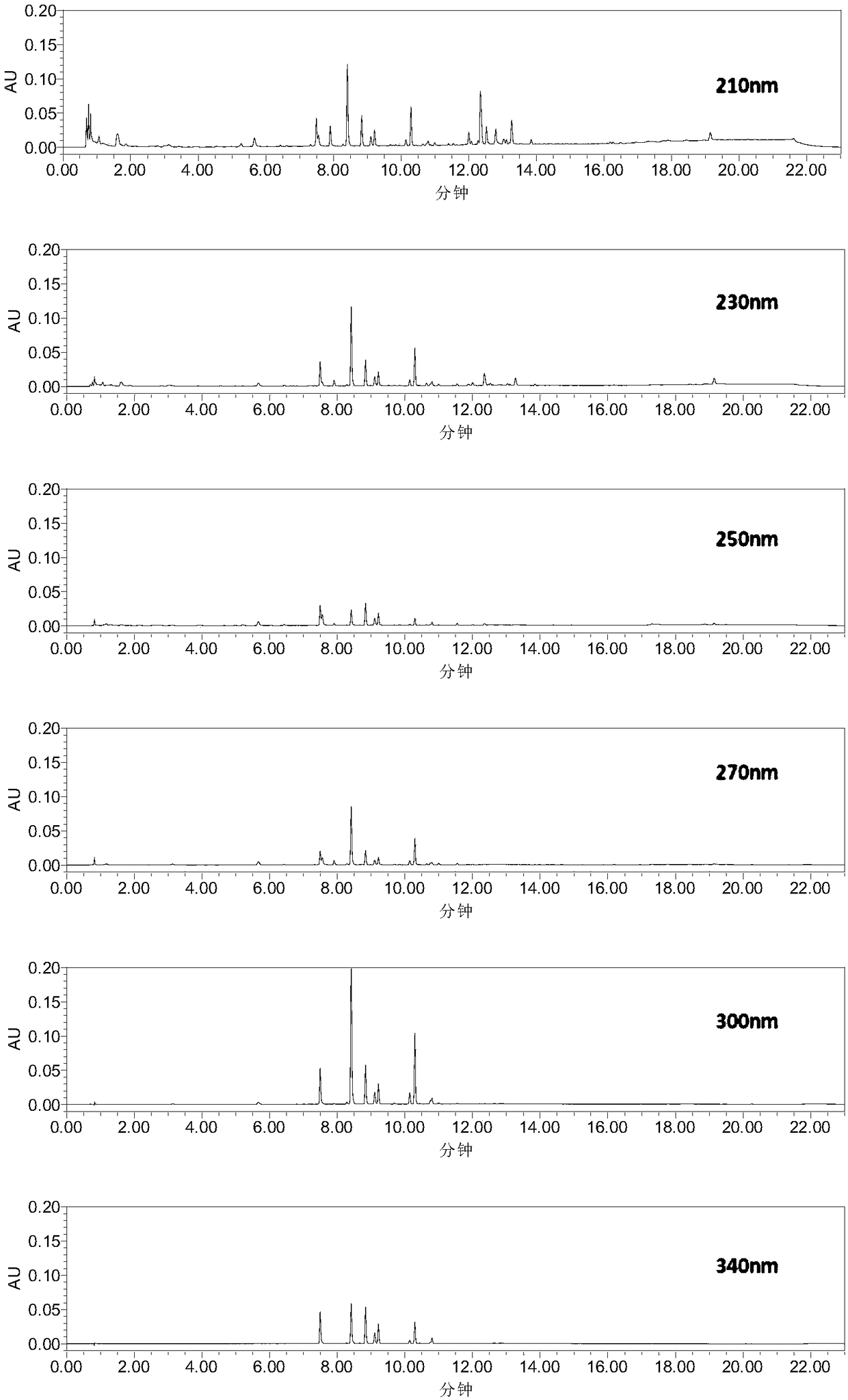
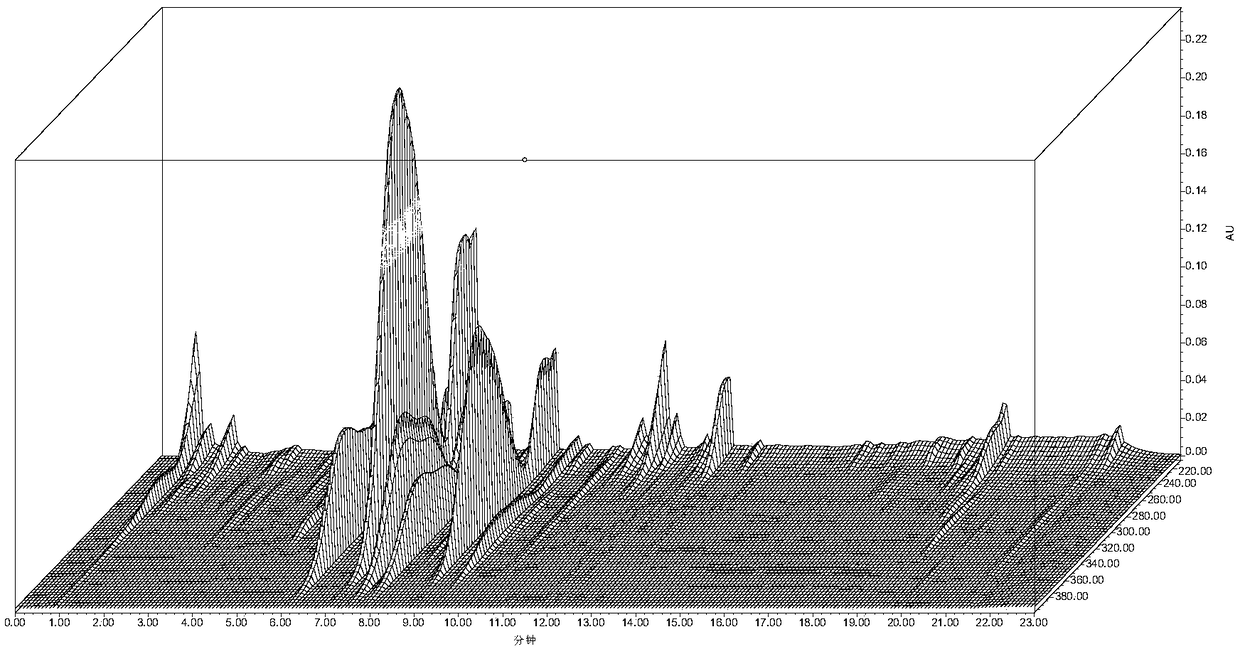
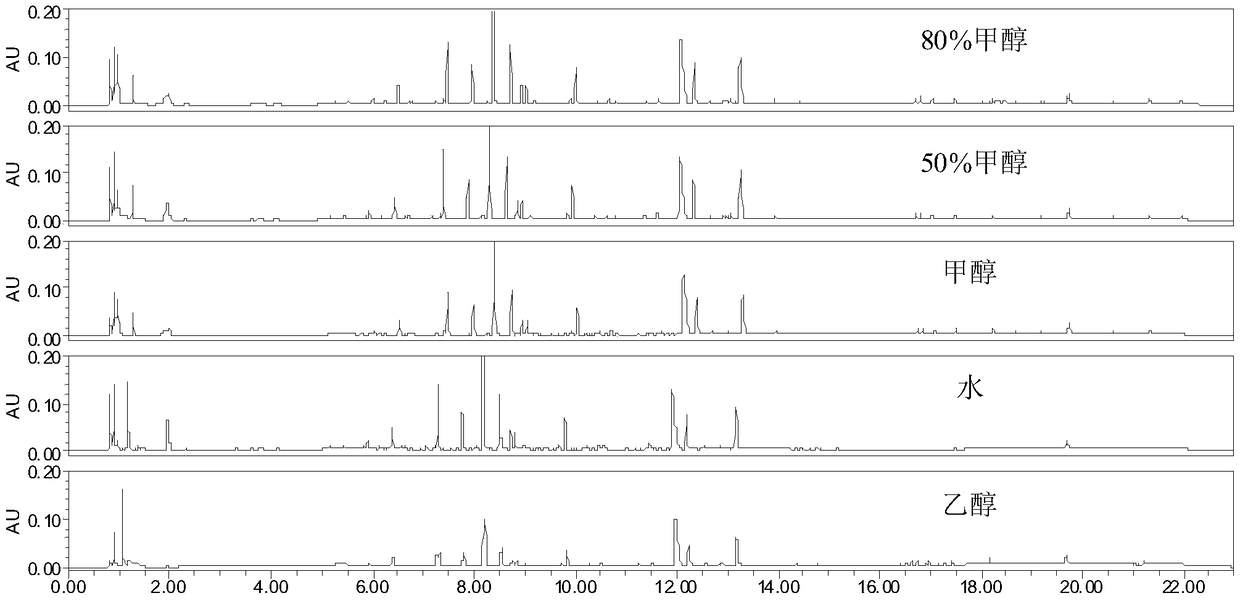
Construction method and detection method of UPLC characteristic chromatogram of stemona tuberosa medicinal material
A technology of Ye Baibu and its construction method, applied in the field of medicine, can solve the problems of not being able to fully reflect the quality, difficult to fully reflect the quality characteristics of Ye Baibu, unable to reflect the material basic characteristics of traditional Chinese medicine decoction, etc., so as to overcome the durability deviation, Ensuring high-quality, characteristic results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Construction of the UPLC characteristic map of the Herb Alba
[0069] 1. Instruments, reagents and reagents
[0070] See Table 1 for instruments, Table 2 for reagents, and Table 3 for reagents.
[0071] Table 1
[0072]
[0073] Table 2
[0074]
[0075]
[0076] table 3
[0077]
[0078] 2. Source of medicinal materials: In this study, 15 batches of raw medicinal materials were collected, including 6 batches from Yichang, Hubei, 3 batches from Jingmen, Hubei, and 6 batches from Dazhou, Sichuan. Detailed source: slightly. The details are shown in Table 4:
[0079] Table 4
[0080]
[0081] Remarks: The content of chlorogenic acid is determined by a self-designed content determination method.
[0082] 3. Preparation of reference substance reference solution, reference medicinal material reference solution and standard decoction
[0083] (1) Take chlorogenic acid reference substance: take an appropriate amount of chlorogenic acid reference...
Embodiment 2
[0200] Embodiment 2 is to the detection method of Herba Abaculi medicinal material
[0201] Stemona sessilifolia (Miq.) Miq., Stemona japonica (Bl.) Miq. or Stemona tuberose Lour. are dry tuberous roots of plants of the family Liceaceae. The present embodiment provides a kind of identification method to the Radix Albae medicinal material, and its steps are as follows:
[0202] (1) The chromatographic conditions are the same as the above 4(1).
[0203] (2) Preparation of the sample solution to be tested
[0204] The sample to be tested is the reference medical material purchased from the China Calibration Research Institute to purchase the Baibu chinensis medicinal material. Take 0.25g of the above-mentioned Baibu medicinal material powder, accurately weigh it, put it in a 10ml volumetric flask, add an appropriate amount of 80% (volume fraction) methanol aqueous solution, and ultrasonically (power 500W, frequency 40kHz) for 10min, take the extract, let the extract cool down, ...
Embodiment 4
[0208] Embodiment 4 is to the detection method of Rhizoma radiata medicinal material
[0209] The present embodiment provides a kind of method for the detection of the medicinal material of Rhizoma chinensis, the steps are as follows:
[0210] (1) The chromatographic conditions are the same as the above 4(1).
[0211] (2) Preparation of the sample solution to be tested
[0212]The samples to be tested are 9 batches of medicinal materials (BB01B1~BB06B1, BB16B1~BB18B1). Take 0.25g of the above 9 batches of medicinal material powders, weigh them accurately, put them in a 10ml volumetric flask, add an appropriate amount of 80% (volume fraction) methanol aqueous solution, and ultrasonically ( Power 500W, frequency 40kHz) for 10min, take the extract, let the extract cool, use 80% (volume fraction) methanol aqueous solution to set the volume to the mark, shake well, centrifuge (8000rpm, 3min), get the supernatant, The supernatant was filtered with a 0.22um microporous membrane, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com