Phenylboronic acid polymer based on amino epoxy group ring-opening polymerization and its preparation method and application
An aminophenylboronic acid and polymer technology, which is applied in the field of phenylboronic acid polymers to achieve the effects of high capacity, high affinity and specific adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
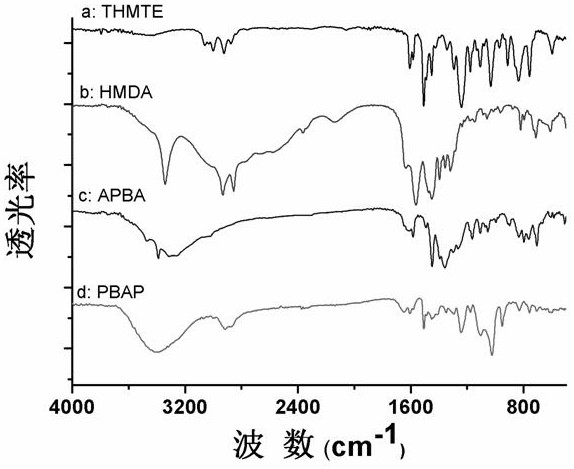
[0030] The hydrophobic phenylboronic acid polymer was prepared by amino-epoxy ring-opening polymerization, and the phenylboronic acid polymer was analyzed and characterized by scanning electron microscope, infrared characterization and nitrogen adsorption analysis. The specific operation steps are as follows:
[0031] The preparation method of phenylboronic acid polymer: dissolving m-aminophenylboronic acid (1.5%, both in mass fraction), tris(4-hydroxyphenyl)methane triglycidyl ether (10.5%) and hexamethylenediamine (3%) in In the mixed solution of dimethyl sulfoxide (25%) and polyethylene glycol 200 (60%), after ultrasonic (ultrasonic electric power 150 watts) mixing, seal in a glass bottle, and react at 60°C for 24-24.5 hours. After the polymerization reaction was completed, the polymer was taken out, ground with a mortar, and unreacted substances were removed by Soxhlet extraction for 48 hours (eluent: dimethyl sulfoxide and methanol). Finally, vacuum-dry the obtained produ...
Embodiment 2
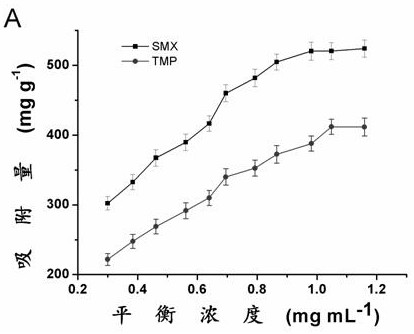
[0039] Standard solution configuration: using methanol and water / dimethyl sulfoxide (95 / 5, volume ratio) as solvents to prepare concentrations of 500, 600, 700, 800, 900, 1000, 1100, 1200, 1300, 1400 and 15000 μg mL -1 Sulfamethoxazole and trimethoprim solution.
[0040] Adsorption: Accurately weigh about 0.01 grams ( m ) of phenylboronic acid polymer into 10 mL centrifuge tubes, and then add 8 mL of different concentrations of sulfamethoxazole or trimethoprim standard solution to each centrifuge tube ( C i ). Shake overnight, centrifuge, and check the concentration of the supernatant by high performance liquid chromatography.
[0041] The equilibrium adsorption capacity was calculated according to the following formula ( Q e ):
[0042] Q e =8( C i - C e ) / m
[0043] Q e To balance the adsorption capacity, C i and C e is the solution concentration of sulfamethoxazole or trimethoprim before and after adsorption, m Is the mass of phenylboronic acid polymer...
Embodiment 3
[0051] Preparation of amino magnetic spheres: using 1.7 grams of FeCl 3 ·6H 2 O, add 3.3 grams of sodium acetate as a dispersant, mix well in 50 mL of ethylene glycol solution, add 10.8 grams of 1,6-hexamethylenediamine, then move the solution into a closed heating container, and carry out the solvent at 200 ° C Thermal reaction, heating time 4-4.5h, to obtain amino magnetic balls.
[0052] The preparation method of phenylboronic acid polymer-wrapped nano-magnetic beads: m-aminophenylboronic acid (1.0%, both mass fractions), tris(4-hydroxyphenyl)methane triglycidyl ether (6.0%), hexamethylenediamine ( 1%) and amino magnetic balls (2%) were dissolved in the mixed solution of dimethyl sulfoxide (30%) and polyethylene glycol 200 (60%), after ultrasonic mixing, as for the three-necked bottle, mechanically The reaction was stirred for 24-24.5 hours. After the completion of the polymerization reaction, the phenylboronic acid polymer-wrapped nano-magnetic beads were separated by a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com