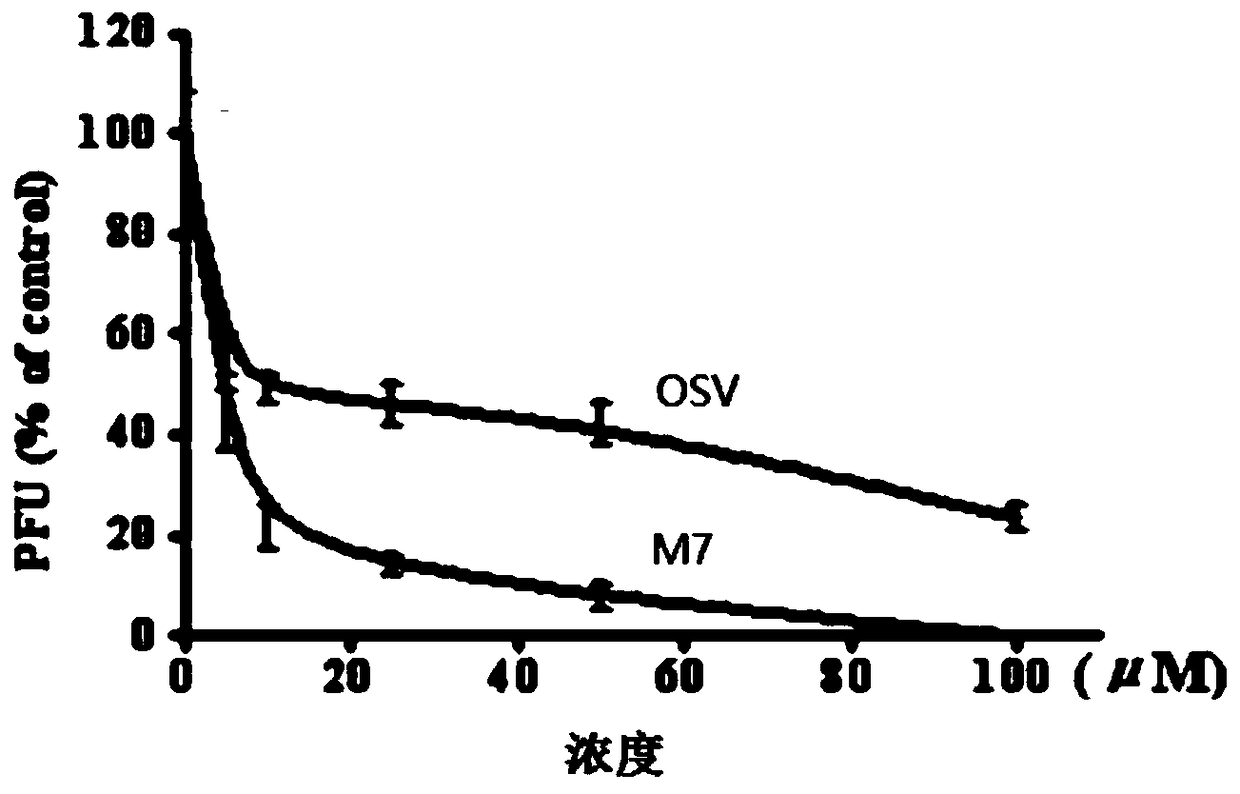
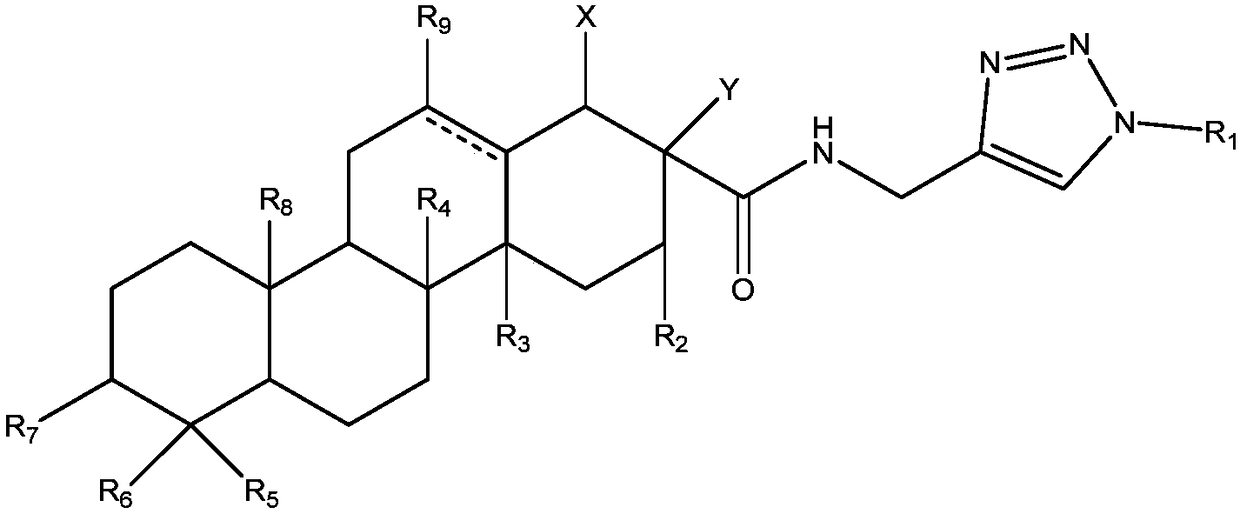
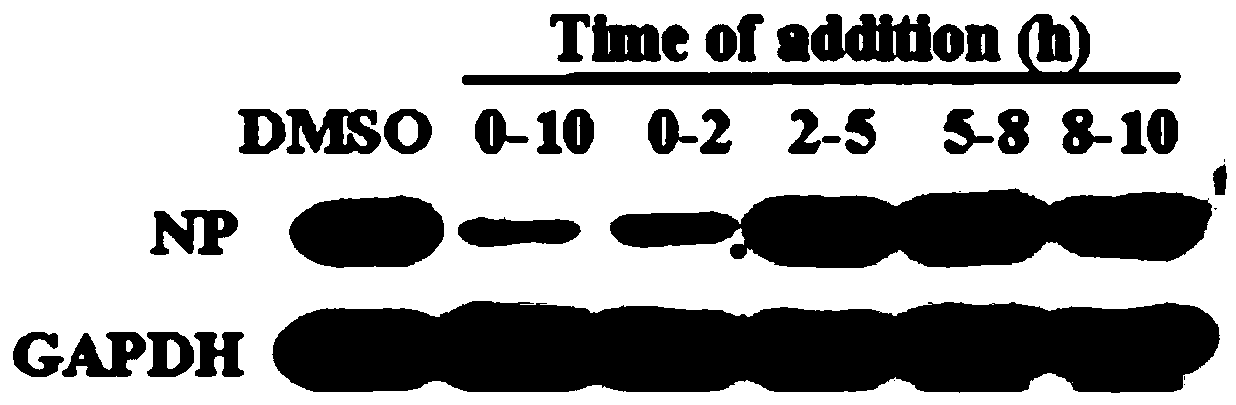Triterpene-oligosaccharide conjugates and application thereof
A technology of conjugates and oligosaccharides, used in steroids, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: The preparation method of this triterpene-oligosaccharide conjugate, taking the preparation of M8 compound as an example, the synthetic route is as follows:
[0064]
[0065] The concrete preparation technology of M8 compound is as follows:
[0066] (a) Take 1g of L-rhamnose in a 100mL reaction bottle, add 30mL of pyridine to dissolve it, then place the reaction bottle in an ice bath, slowly add 10.5mL of acetic anhydride with a normal pressure dropping funnel, and transfer to Reaction at room temperature, TLC detection after 3h, developer petroleum ether: ethyl acetate = 1:1, CMC chromogenic reagent for color development. Pyridine and excess acetic anhydride were directly evaporated to dryness to obtain compound M30; the crude product could be directly used in subsequent reactions without purification, but it was necessary to ensure that the product point on the thin layer was single, and if the product point was not single, purification by column chromat...
Embodiment 2
[0097] Embodiment 2: The compound of the present invention inhibits the biological activity evaluation method of influenza virus entering cells
[0098] 1. Cytopathic Efficacy (CPE) Inhibition Test
[0099] After influenza virus infects cells, it will cause cell pathology, which will reduce cell viability; if the drug can inhibit the replication of influenza virus, it will reduce the number of cell lesions and increase cell viability; the specific method is as follows:
[0100] (1) Canine kidney epithelial cells (MDCK) were passaged into a white 96-well plate at a ratio of 1:3, and cultured in DMEM medium containing 10% FBS for 24 hours in a cell culture incubator at 37°C;
[0101] (2) Add influenza virus [A / WSN / 33 (H1N1), multiplicity of infection (MOI) = 1] and 100 μM / L of the compound to be tested to 100 μl of DMEM containing 2 μg / mL TPCK-treated trypsin and 1% FBS Mix well; the negative control of the compound is 1% DMSO (the solvent used for diluting the compound); set u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com