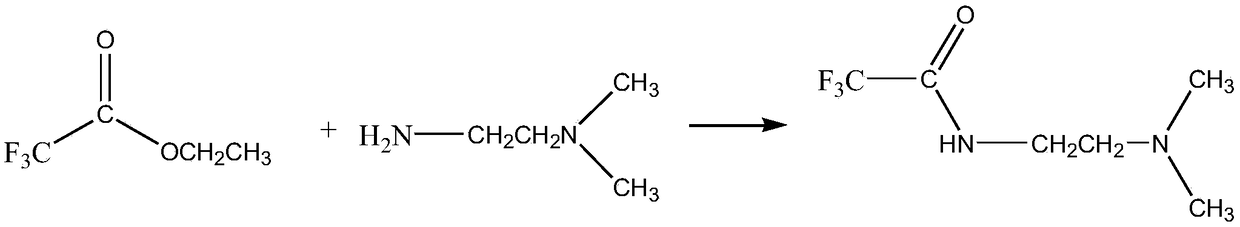
Fluorine-containing amide and preparation method thereof
A technology containing fluorine-containing amides and dimethylethylenediamine, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds. The effect of short operation and process cycle and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific steps. The present invention discloses a fluorine-containing amide and a preparation method thereof. The preparation method specifically includes the following steps:
[0024] Step 1: Take N,N-dimethylethylenediamine and add it to distilled water, the mixing ratio is 3mol:2L, and after fully stirring, an aqueous solution of N,N-dimethylethylenediamine is obtained;
[0025] Step 2, take ethyl trifluoroacetate which is equimolar to N,N-dimethylethylenediamine, and slowly add it dropwise to N,N-dimethylethylenediamine aqueous solution using a constant pressure dropping funnel, The dropping time is 0.5-1h to obtain a reaction system;
[0026] Step 3, the reaction system obtained in step 2 is reacted at 0-30°C for 2-4 hours, and solution A is obtained after the reaction is completed;
[0027] In step 4, the solution A was distilled under reduced pressur...
Embodiment 1
[0034] Step 1: Take 0.03mol of N,N-dimethylethylenediamine and add it to 0.02L of distilled water, and stir thoroughly to obtain an aqueous solution of N,N-dimethylethylenediamine;
[0035] Step 2, take 0.03 mol of ethyl trifluoroacetate, and slowly add it dropwise to the aqueous solution of N,N-dimethylethylenediamine using a constant pressure dropping funnel, and the dropping time is 0.5h to obtain a reaction system;
[0036] Step 3, the reaction system obtained in step 2 was reacted at 10°C for 3 hours, and solution A was obtained after the reaction was completed;
[0037] In step 4, the solution A was distilled under reduced pressure and freeze-dried to obtain yellow crystals, namely N-[2-(dimethylamino)ethyl]-2,2,2-trifluoroacetamide.
Embodiment 2
[0039] Step 1: Add 3 mol of N,N-dimethylethylenediamine into 2L of distilled water, and stir thoroughly to obtain an aqueous solution of N,N-dimethylethylenediamine;
[0040] Step 2: Take 3 mol of ethyl trifluoroacetate, and slowly add it dropwise to the aqueous solution of N,N-dimethylethylenediamine using a constant pressure dropping funnel, and the dropping time is 1 hour to obtain a reaction system;
[0041] Step 3, the reaction system obtained in step 2 was reacted at 30°C for 2 hours, and solution A was obtained after the reaction was completed;
[0042] In step 4, the solution A was distilled under reduced pressure and freeze-dried to obtain yellow crystals, namely N-[2-(dimethylamino)ethyl]-2,2,2-trifluoroacetamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com