A kind of ivermectin derivative and anti-abamectin monoclonal antibody and application thereof
A monoclonal antibody, abamectin technology, applied in the direction of sugar derivatives, chemical instruments and methods, instruments, etc., can solve the problems of poor broad-spectrum antibodies, single type of detection samples, etc., and achieve low detection cost and high accuracy. , the effect of small health hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of immunogen and coating original
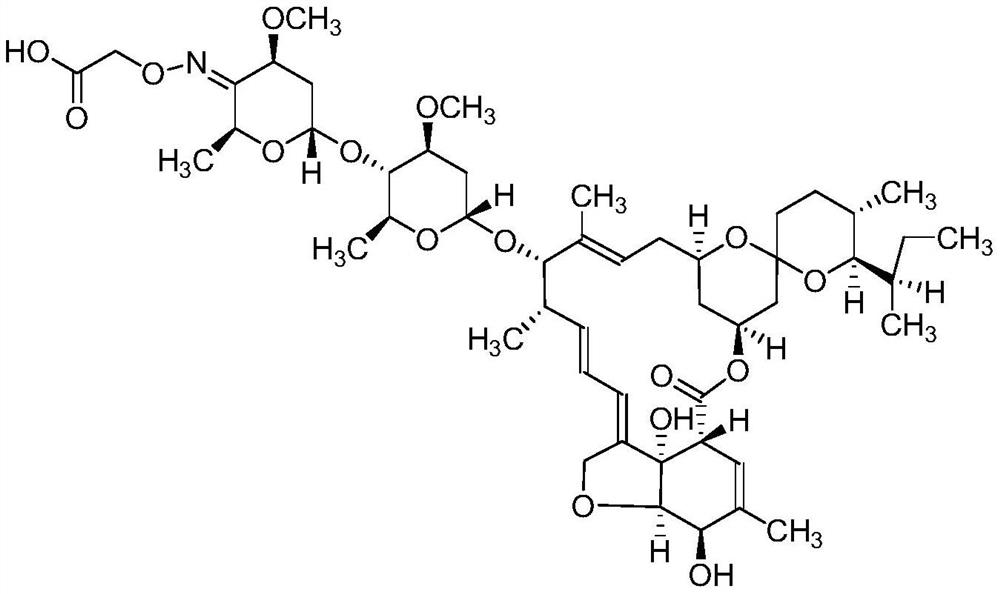
[0033] 1.1 Preparation of hapten 4"-CMO-IVM
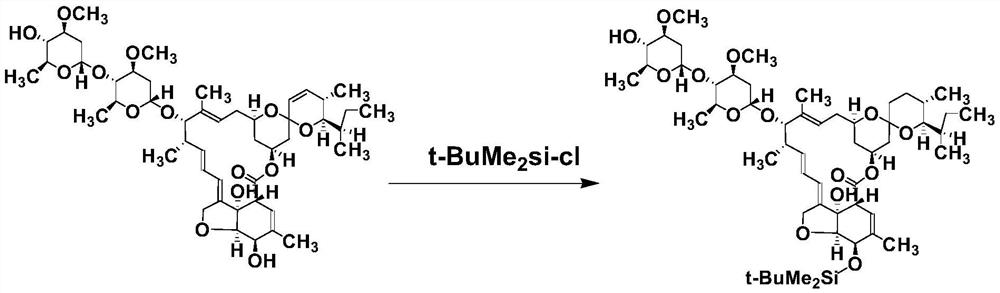
[0034] (1) 5′-O-t-BuMe 2 Synthesis of Si-IVM: 4.0 g of IVM was placed in a 50 mL round bottom flask, 20 mL of tetrahydrofuran was added to dissolve it, and 1.8 g of imidazole was added and mixed. Under mechanical stirring, dissolve 2.0gt-BuMe in 10 mL of tetrahydrofuran 2 SiCl was added dropwise and reacted at 30°C for 4h. The reaction was monitored by TLC. After the reaction was completed, 100 mL of ethyl acetate was added to the reaction solution and mixed. The mixed solution was washed three times with water to separate the ethyl acetate layer, and the organic layer was collected. 4 It was dried and concentrated under reduced pressure to give a yellowish viscous substance. The residue was dissolved in an appropriate amount of ethyl acetate, and the product was separated by silica gel column chromatography (eluent ethyl acetate: n-hexane = 1:1, v / v), the second ...
Embodiment 2
[0044] Example 2 Preparation of monoclonal antibodies
[0045] 2.1 Animal immunization
[0046] The immunogen 4"-CMO-IVM-KLH prepared by the inventor's national veterinary drug residue benchmark laboratory was used to immunize female Balb / C mice (purchased from the Laboratory Animal Center of Hubei Provincial Center for Disease Control and Prevention). The immunization procedure was to take the immunogen 4"-CMO-IVM-KLH was mixed with an equal amount of Freund's adjuvant at a protein content of 50 μg and injected into mice to produce specific serum.
[0047] The immunization procedure was as follows: the immunogen was emulsified with an equal volume of Freund's complete adjuvant for the basic immunization, and then subcutaneously injected into the back of the mice at multiple points. After that, the immunization was boosted every 2 weeks, and the incomplete Freund's adjuvant was used for emulsification. Finally, intraperitoneal injection was given three days before fusion (pre...
Embodiment 3I
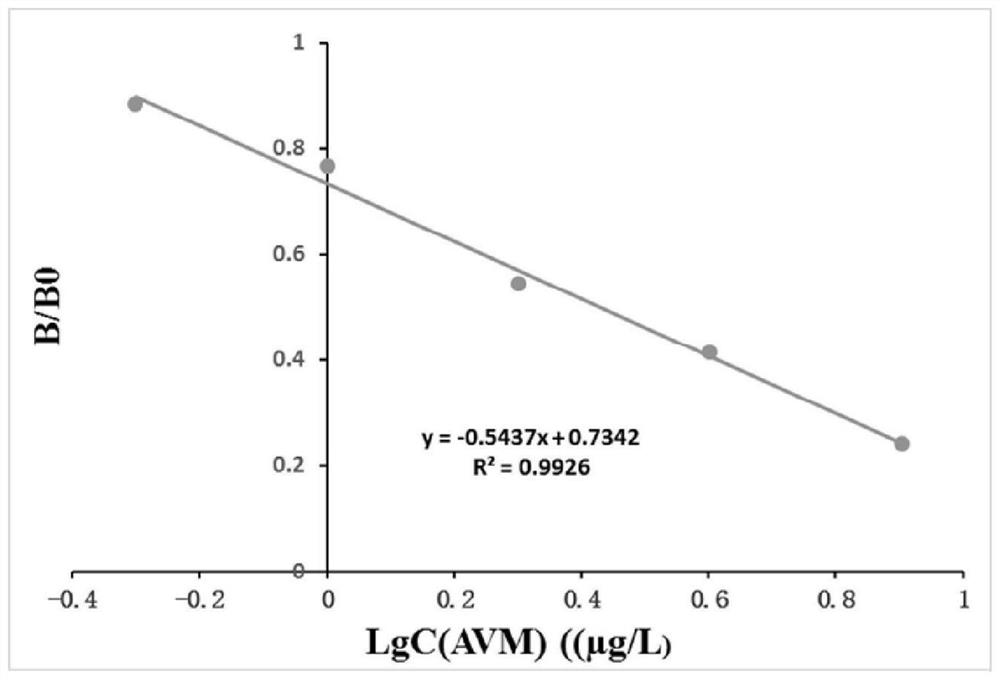
[0051] Example 3 Establishment of IVM Indirect Competitive ELISA Detection Method
[0052] 3.1 Preparation of reagents (reagents used in this example were prepared by the following methods unless otherwise noted)
[0053] AVM standard stock solution: Weigh a bottle of 5 mg AVM standard product, add 5 mL of methanol to dissolve, and vortex for 2 min, that is, the mother solution of 1 mg / mL.
[0054] Phosphate buffer (pH 7.4): accurately weigh NaCl 8.00g, KH 2 PO 4 0.20g, Na 2 HPO 4 ·12H 2 O2.90g, KCl 0.20g, dissolve in a small amount of deionized water, and dilute to 1000mL.
[0055] Phosphate buffer (pH 8): accurately weigh KH 2 PO 4 0.41g, K 2 HPO 4 5.59g, dissolve in a small amount of deionized water, and dilute to 1000mL.
[0056] Carbonate buffer (pH 9.6): accurately weigh Na 2 CO 3 1.59g, NaHCO 3 2.93g, dissolve in a small amount of deionized water, and dilute to 1000mL.
[0057] Coating solution: take Na 2 CO 3 1.59g, NaHCO 3 2.93g, add three dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com