Preparation of immunizing antigens and envelope antigens for detecting furazolidone metabolites
The technology of furazolidone and immunization antigen is applied in the field of preparation of immunization antigen and coated antigen, which can solve the problems of residual metabolites and the like, and achieve the effects of improving immunogenicity, improving specificity and improving coupling ratio.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
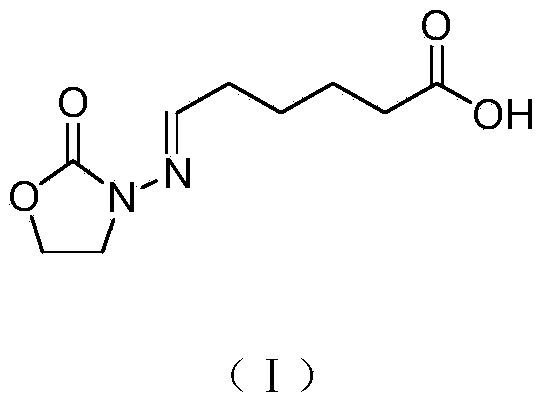
[0020] 1 Synthesis of furazolidone metabolite hapten by acetal method
[0021] 200 mL of ethanol was added into a 500 mL round bottom flask, furazolidone (10.2 g, 0.1 mol) and 6-oxohexanoic acid (15.6 g, 0.12 mol) were added in sequence, and heated to reflux for 3 hours. Heating was stopped, and the reaction solution was naturally cooled to room temperature, and left to stand overnight. After suction filtration, the filter cake was washed with ethanol (20.0 mL×3), and dried at 50° C. for 5 hours to obtain 20.4 g of carboxyl-containing furazolidone derivatives. Preparation of furazolidone metabolite hapten (I).
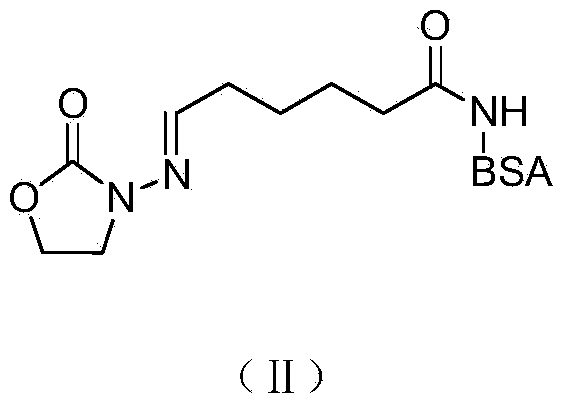
[0022] 2 Synthesis of Furazolidone Metabolite Immune Antigen by Active Thioester Method
[0023] Dissolve the furazolidone hapten (0.214g, 1.0mmol) in 30mL of dimethylformamide (Nimethylformamide, DMF), cool to 0-4°C, add 3-phosphorylated diphenyl ester benzo[D]oxane under stirring Azol-2-one [2(3H)-Benzoxazolone,3-(diphenoxyphosphinyl)-(9CI), DPBO] (0.351g, 1.0mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com