Preparation method and application of attenuated live vaccine chitosan microsphere for porcine epidemic diarrhea virus
A technology for porcine epidemic diarrhea and a live attenuated vaccine, which is applied in the field of biomedicine, can solve the problems of decreased effect, can not provide protection, can not effectively prevent diarrhea, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method of porcine epidemic diarrhea virus attenuated live vaccine chitosan microspheres comprises the following steps:
[0048] 1) Cultivate the attenuated strain of porcine epidemic diarrhea virus PEDV-YN144 to obtain the virus liquid; then centrifuge and filter to remove cell debris and other impurities to obtain the virus filtrate, wherein the virus content in the virus filtrate is greater than or equal to 10 6 TCID 50 / ml, and less than or equal to 10 8 TCID 50 / ml;
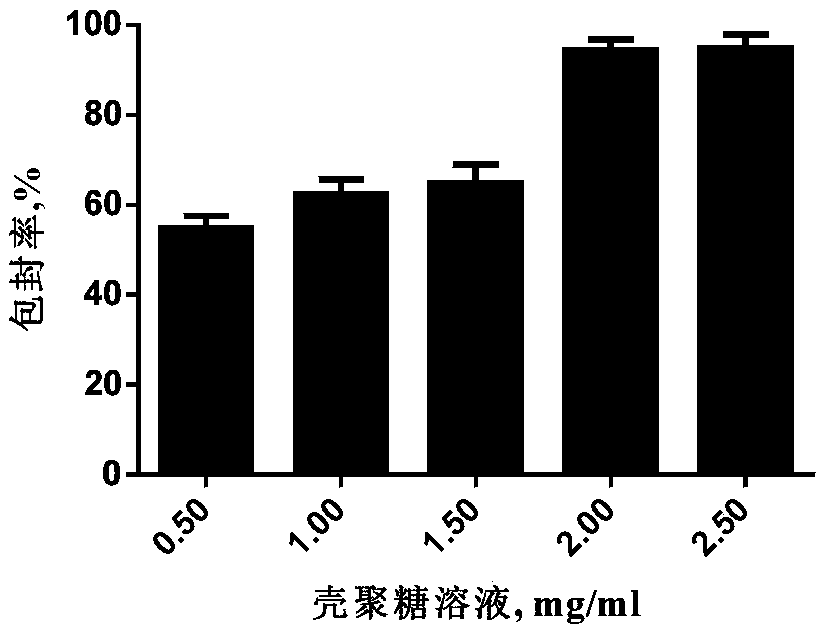
[0049] 2) adding chitosan to an acetic acid solution with a volume concentration of 1% to 5%, and ultrasonically dissolving it to obtain a chitosan solution; wherein, the concentration of the chitosan solution is 0.5 to 5 mg / 1ml;
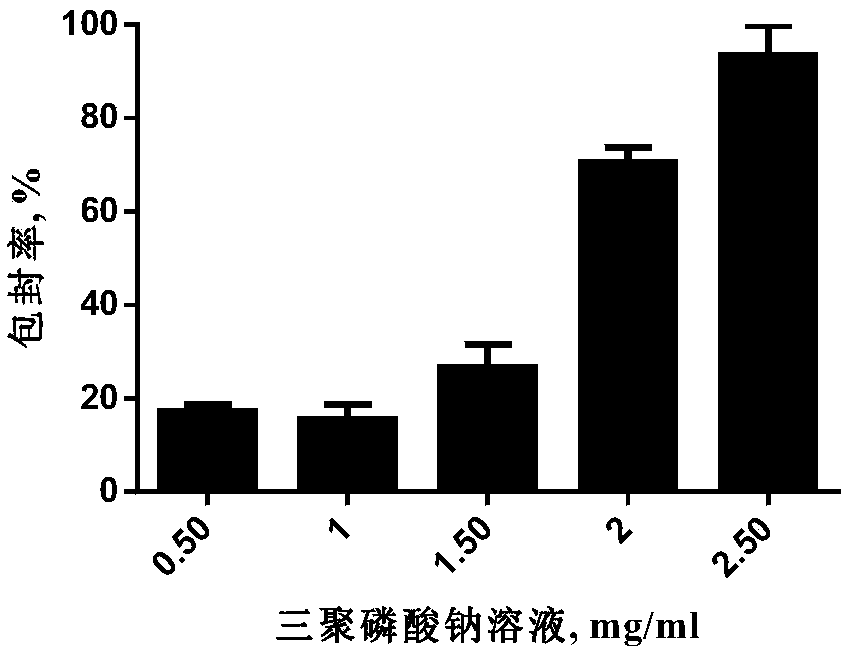
[0050] 3) measure chitosan solution, virus filtrate, sodium bicarbonate solution and sodium tripolyphosphate solution by volume ratio 3: 3~20: 0.060~0.070: 1~20; Wherein, the concentration of sodium bicarbonate solution is 60~ 100mg / ml, the concentration ...
Embodiment 1
[0070] The preparation method of porcine epidemic diarrhea virus attenuated live vaccine chitosan microsphere 1 comprises the following steps:
[0071] 1) Cultivate porcine epidemic diarrhea virus attenuated strain PEDV-YN144 to obtain virus liquid; then centrifuge and filter to remove cell debris and other impurities to obtain virus filtrate, wherein the virus content in the virus filtrate is equal to 10 6 TCID 50 / ml;
[0072] 2) adding chitosan to an acetic acid solution with a volume concentration of 3%, and ultrasonically dissolving; that is, obtaining a chitosan solution; wherein, the concentration of the chitosan solution is 3 mg / 1ml;
[0073] 3) measure the chitosan solution of 3ml, the virus filtrate of 15ml, the sodium bicarbonate solution of 0.067ml and the sodium tripolyphosphate solution of 1ml; Wherein, the concentration of sodium bicarbonate solution is 96mg / ml, the sodium bicarbonate solution The concentration is 0.5mg / ml;
[0074] 4) To the sodium bicarbona...
Embodiment 2
[0077] The preparation method of porcine epidemic diarrhea virus attenuated live vaccine chitosan microsphere 2 comprises the following steps:
[0078] 1) Cultivate porcine epidemic diarrhea virus attenuated strain PEDV-YN144 to obtain virus liquid; then centrifuge and filter to remove cell debris and other impurities to obtain virus filtrate, wherein the virus content in the virus filtrate is equal to 10 6 TCID 50 / ml;
[0079] 2) adding chitosan to an acetic acid solution with a volume concentration of 3%, and ultrasonically dissolving; that is, obtaining a chitosan solution; wherein, the concentration of the chitosan solution is 3 mg / 1ml;
[0080] 3) measure the chitosan solution of 3ml, the virus filtrate of 7ml, the sodium bicarbonate solution of 0.067ml and the sodium tripolyphosphate solution of 1ml; Wherein, the concentration of sodium bicarbonate solution is 96mg / ml, the sodium bicarbonate solution The concentration is 1.0mg / ml;
[0081] 4) To the sodium bicarbonate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com