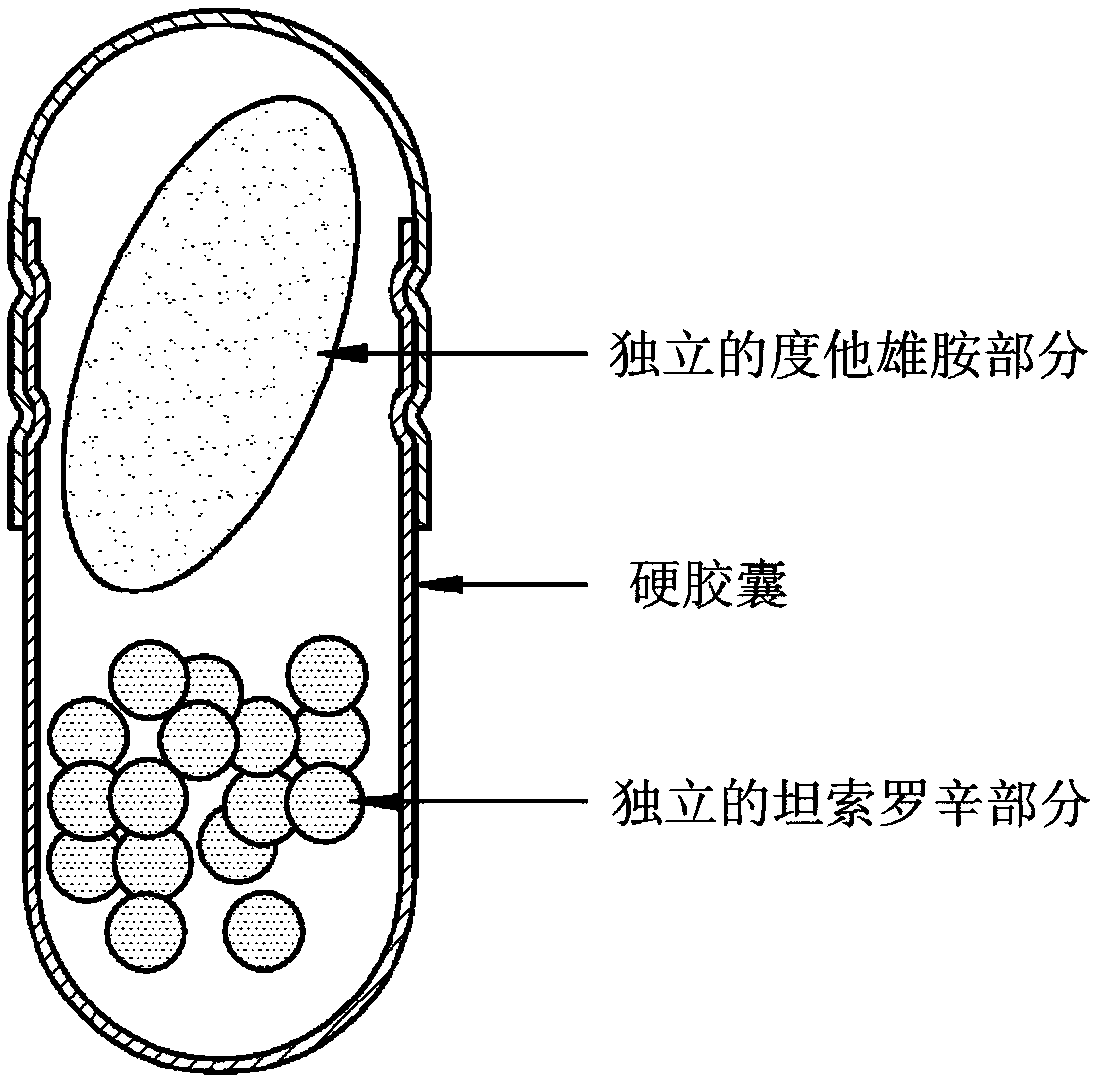
Dutasteride- and tamsulosin-containing hard capsule complex and preparation method therefor
A technology of dutasteride and hard capsules, applied in medical preparations containing active ingredients, capsule delivery, microcapsules, etc., can solve the problems of poor drug compliance, reduce the rapid action of dutasteride, etc., and achieve fast drug effect , The effect of improving drug compliance and high initial dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: prepare hard capsule compound preparation (1)
[0095] Prepare a separate dutasteride portion and a separate tamsulosin portion according to the following prescription:
[0096] -Independent dutasteride moiety-
[0097]
[0098] -Separate Tamsulosin section-
[0099]
[0100] Separate dutasteride fractions were prepared by dissolving dutasteride in monoglyceride oil and diglyceride oil, adding thereto all remaining components, and dissolving the mixture to prepare a self-emulsifying emulsion. The prepared self-emulsifying emulsion was filled into soft capsules to prepare dutasteride soft capsules. In detail, the coating composition was prepared using a capsule base material having a weight ratio of 81:46:1 gelatin:glycerol:glycine according to a conventional method for preparing a coating. Next, according to the conventional filling method, use the rotary automatic filling machine to fill the prepared self-emulsifying emulsion into 2 circles and...
Embodiment 2
[0103] Embodiment 2: prepare hard capsule compound preparation (2)
[0104]Tamsulosin granules were prepared in the same manner as in Example 1, which were then further mixed with 9 mg (5%) of crospovidone as a disintegrant, followed by tableting by a circular punch with a diameter of 6.5 mm . The obtained tamsulosin tablets and dutasteride soft capsules were filled into size 0 gelatin hard capsules (SUHEUNG Co., Ltd.) to prepare a capsule composite preparation.
Embodiment 3
[0105] Embodiment 3: prepare hard capsule compound preparation (3)
[0106] An independent dutasteride part was prepared in the same manner as in Example 1, and an independent tamsulosin part was prepared by the following prescription:
[0107] -Separate Tamsulosin section-
[0108]
[0109] Separate tamsulosin fractions were prepared by mixing all listed tamsulosin hydrochloride, microcrystalline cellulose, hypromellose and 6.0 mg talc in powder form and using A binder solution prepared by dissolving acrylic acid-ethyl acrylate copolymer in water to prepare particles. Next, the prepared tamsulosin granules were coated with an enteric coating solution by dissolving 7.0 mg of methacrylic acid-ethyl acrylate copolymer, triacetin and 3.0 mg of talc in prepared in water to prepare tamsulosin hydrochloride granules. Sucrose stearate was added thereto and mixed therewith to prepare tamsulosin granules.
[0110] The obtained tamsulosin granules and dutasteride soft capsules ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com