Alkyl and heterocyclic compound serving as hepatitis-c-virus inhibitor and application of alkyl and heterocyclic compound in medicine
A compound and solvate technology, applied in the field of hepatitis C virus inhibitors and applications, can solve problems such as inability to treat for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] In another preferred example, the preparation method provided by the present invention includes the steps of:
[0088] (a) in an inert solvent, compound 1 and reacting to form compound 2;
[0089]
[0090] (b) reacting compound 2 and benzophenone imine in an inert solvent to form compound 3;
[0091]
[0092] (c) reacting compound 3 with an acid in an inert solvent to form compound 4;
[0093]
[0094] (d) In an inert solvent, compound 4 and reacting to form compound 5;
[0095]
[0096] (e) reacting compound 5 with an acid in an inert solvent to form compound 6;
[0097]
[0098] (f) in an inert solvent, compound 6 and reacting to form a compound of formula (I);
[0099]
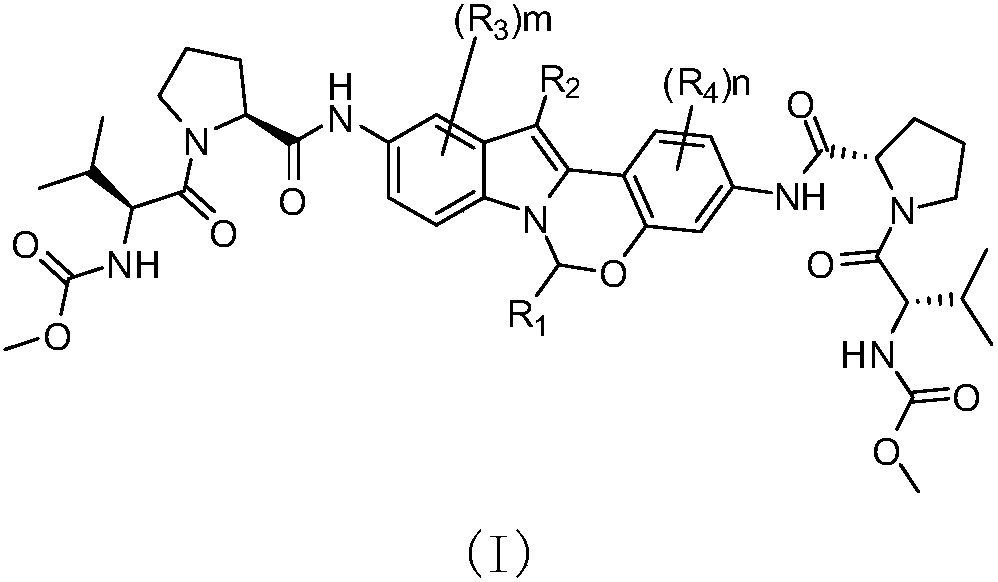
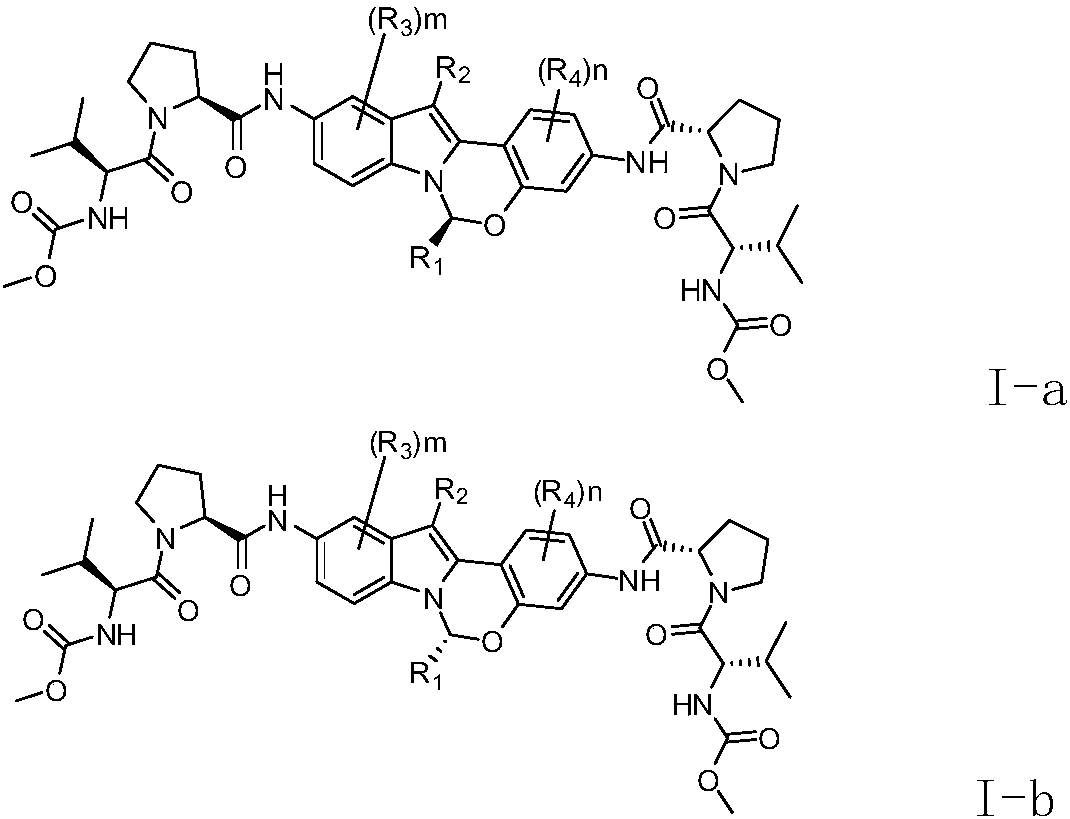
[0100] In various forms, R 1 , R 2 , R 3 , R 4 , m and n are defined the same as before.
[0101] In another preferred example, the inert solvent used in the preparation method of the present invention is selected from the following group: acetic acid, ethanol, N,N-dim...
Embodiment 1
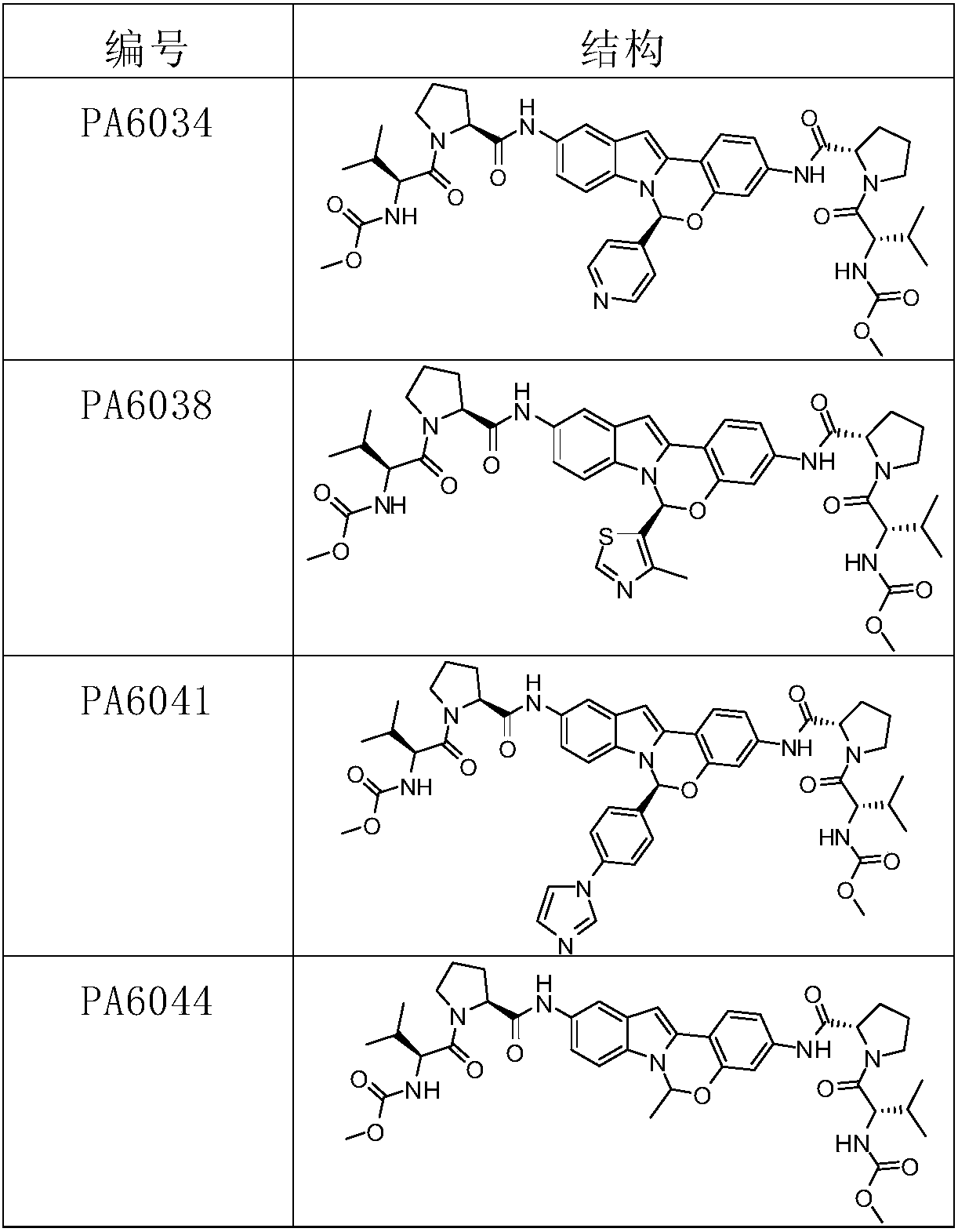
[0136] Example 1: PA6034
[0137] synthetic route:
[0138]
[0139] Experimental part:
[0140] Step 1) Synthesis of compound PA6034-1:
[0141] Compound PA6016-B-6 (1.17g, 3mmol) was dissolved in acetonitrile (15ml), and 4-formaldehyde pyridine (0.48g, 4.5mmol) and trifluoroacetic acid (0.1g) were added at room temperature, under nitrogen protection, and the reaction solution was heated Stir at 35°C for 3 hours, cool to room temperature, and suction filter to obtain a crude solid (1.05g) of compound PA6034-1 with a yield of 77%, which is directly used for the next step.
[0142] Step 2) Synthesis of compound PA6034-2:
[0143] Compound PA6034-1 (1.0g, 2.15mmol), DDQ (0.73g, 3.22mmol) was dissolved in toluene (15ml), and the reaction solution was stirred overnight at 110°C. After the reaction was complete, it was suction filtered, the filtrate was spin-dried, and saturated saline ( 20mL), extracted with ethyl acetate (3×25mL), combined the organic phases, washed with s...
Embodiment 2
[0154] Example 2: PA6038
[0155] synthetic route:
[0156]
[0157] Experimental part:
[0158] Step 1) Synthesis of compound PA6038-1:
[0159] Compound PA6016-B-6 (3.73g, 10mmol) was dissolved in acetonitrile (20ml), and 4-methylthiazole-5-carbaldehyde (1.27g, 10mmol) and trifluoroacetic acid (1.2mL) were added at room temperature, nitrogen protection , stirred overnight at room temperature, added saturated sodium carbonate solution (50mL) and water (50mL) to the reaction solution, stirred for 0.5 hours, extracted with ethyl acetate (3×100mL), combined organic phases, washed with saturated brine, anhydrous Na 2 SO 4 Dry, remove the solvent under reduced pressure, separate and purify by silica gel column chromatography (eluent: PE:EA(V:V)=3:1) to obtain PA6038-1 (2.23g), yield 46.9%.
[0160] Step 2) Synthesis of compound PA6038-2:
[0161] Compound PA6038-1 (2.07g, 4.3mmol) was dissolved in toluene (50mL), DDQ (2.9g, 12.9mmol) was added, and the reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com