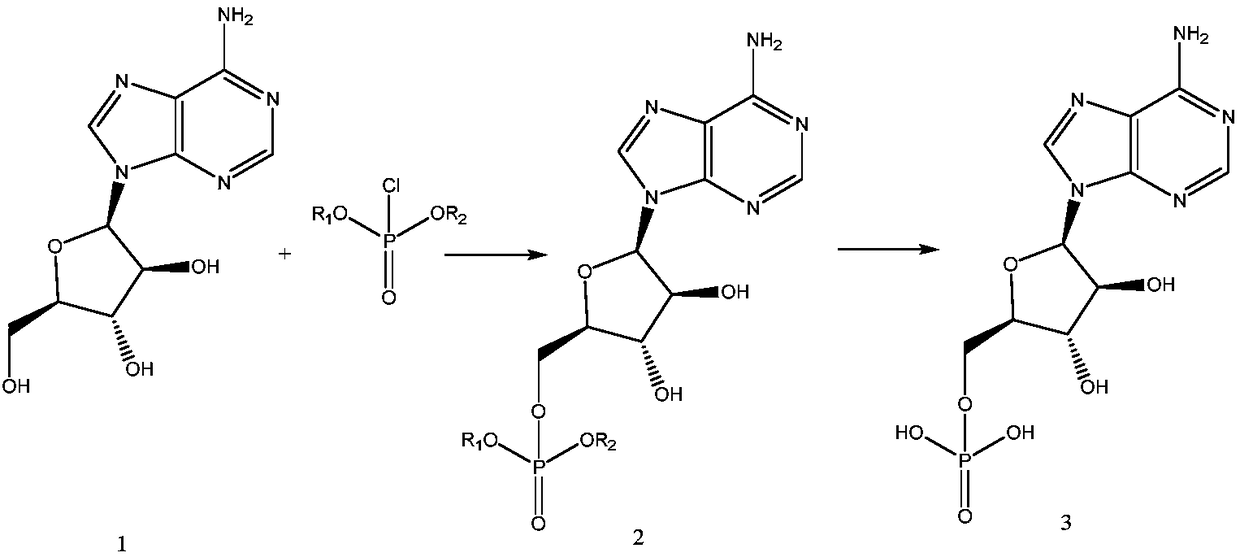
Production process of vidarabine monophosphate
A technology of vidarabine monophosphate and vidarabine monophosphate crude product, which is applied in the direction of preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc. It can solve the problems of expensive solvent acetonitrile and high toxicity of pyridine, etc. Achieve the effects of easy control, high yield and purity, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A production process of adenosine monophosphate, which comprises the steps:
[0038] S1 Mix 30 g of adenosine arabinoside in 3 mL of tetrahydrofuran, and stir to cool down to 0 °C;
[0039] S2 adds 30.83g of di-n-butylphosphoryl chloride in batches to the system after the cooling in step S1, and after the addition, the reaction is continued at 10°C until the remaining amount of adenosine vibranium does not exceed 3% of the added amount;
[0040] S3 Add 1.5 g of palladium catalyst to the reaction system after the stop of step S2, and heat up to 50 ° C and stir for 8 h, filter, and wash the filter cake with ethanol, collect the filtrate, remove the solvent under reduced pressure, and obtain the crude product of adenosine monophosphate. ;
[0041] The preparation method of described palladium catalyst comprises the steps:
[0042] Add 17.7 g of palladium chloride and 203.3 g of magnesium chloride hexahydrate to 20 mL of saturated ammonia solution, and stir until complete...
Embodiment 2
[0047] A production process of adenosine monophosphate, which comprises the steps:
[0048] S1 Mix 30 g of adenosine arabinoside in 3 mL of acetone, stir and cool down to -5°C;
[0049] S2 adds 52.12 g of bis(4-nitrophenyl) phosphoryl chloride in batches to the system cooled in step S1, and continues to react at 5°C after the addition until the remaining amount of adenosine does not exceed 3% of the added amount stop;
[0050] S3 Add 1.5 g of palladium catalyst to the reaction system after the stop of step S2, and heat up to 50 ° C and stir for 8 h, filter, and wash the filter cake with ethanol, collect the filtrate, and remove the solvent under reduced pressure to obtain the crude product of adenosine monophosphate. ; The preparation method of described palladium catalyst is with embodiment 1;
[0051]S4 after the crude adenosine monophosphate obtained in step S3 is just dissolved in water at 40°C, a mixed solution of diethyl ether, methanol and triethylamine is gradually a...
Embodiment 3
[0053] A production process of adenosine monophosphate, which comprises the steps:
[0054] S1 Mix 30 g of adenosine arabinoside in 3 mL of dioxane, stir and cool down to 8 °C;
[0055] S2 adds 32.54 g of bis(2-chloroethyl) phosphoryl chloride in batches to the system cooled in step S1, and continues to react at 15°C after the addition until the remaining amount of adenosine does not exceed 1% of the added amount stop;
[0056] S3 Add 1.5 g of palladium catalyst to the reaction system after the stop of step S2, and heat up to 50 ° C and stir for 8 h, filter, and wash the filter cake with ethanol, collect the filtrate, remove the solvent under reduced pressure, and obtain the crude product of adenosine monophosphate. ;
[0057] The preparation method of described palladium catalyst is with embodiment 1;
[0058] S4 After the crude adenosine monophosphate obtained in step S3 is just dissolved in water at 40°C, a mixed solution of ether, methanol and ethyl acetate is gradually...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com