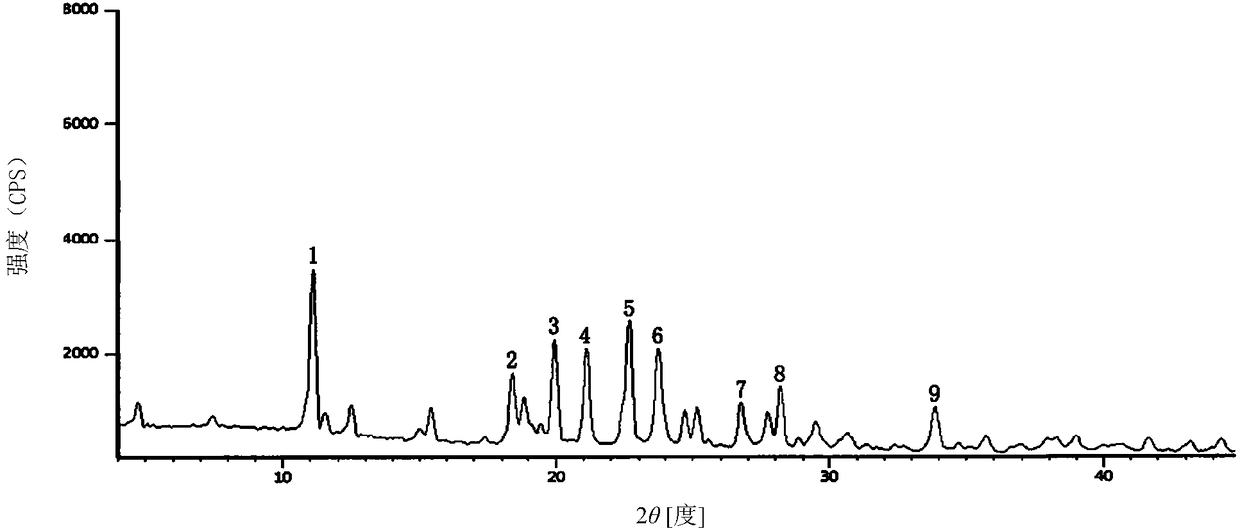
Ceftriaxone sodium tetrahydrate compound
A technology of ceftriaxone sodium and its compound, which is applied in the field of ceftriaxone sodium tetrahydrate compound and its preparation method, and can solve the problems of increased production cost, waste of raw materials, instability between batches, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0035] The preparation of embodiment 1 ceftriaxone sodium tetrahydrate compound
[0036]Preparation Process:
[0037] (1) Slowly add 16.34g of sulfonyl chloride to 8.41g of dimethylformamide, stir well, add 100ml of dichloromethane, stir rapidly, slowly pour in 20.01g of aminothioxime acetic acid, and stir to obtain aminothiazide Clear solution of oxime acetic acid active.
[0038] (2) Add 35.10 g of 7-aminocefatriazine (7-ACT) into 200 ml of dichloromethane, stir well, cool, slowly add 20.01 g of tetramethylguanidine, and stir to obtain 7-ACT tetramethylguanidine salt solution.
[0039] (3) Add the activator solution of aminothioxime acetic acid into the solution of 7-ACT tetramethylguanidine salt for reaction. After adding 200ml of water, raise the temperature to 20°C, adjust the pH value to 6.0 with triethylamine, separate the water layer, add 1.50g of activated carbon and 0.55g of sodium bisulfite, decolorize for 30min, and filter. The filtrate was adjusted to pH 4.2 w...
Embodiment 2 4
[0045] The preparation of embodiment 2 ceftriaxone sodium tetrahydrate compound
[0046] Preparation Process:
[0047] (1) Slowly add 16.15g of sulfonyl chloride to 8.40g of dimethylformamide, stir evenly, add 100ml of dichloromethane, stir rapidly, slowly pour in 20.04g of aminothioxime acetic acid, and stir to obtain aminothiazide Clear solution of oxime acetic acid active.
[0048] (2) Add 35.10 g of 7-aminocefatriazine (7-ACT) into 200 ml of dichloromethane, stir well, cool, slowly add 20.00 g of tetramethylguanidine, and stir to obtain 7-ACT tetramethylguanidine salt The solution.
[0049] (3) Add the activator solution of aminothioxime acetic acid into the solution of 7-ACT tetramethylguanidine salt for reaction. After adding 200ml of water, raise the temperature to 25°C, adjust the pH value to 5.0 with triethylamine, separate the water layer, add 1.51g of activated carbon and 0.54g of sodium bisulfite, decolorize for 30min, and filter. The pH value of the filtrate w...
Embodiment 3 4
[0055] The preparation of embodiment 3 ceftriaxone sodium tetrahydrate compound
[0056] Preparation Process:
[0057] (1) Slowly add 16.21g of sulfonyl chloride to 8.47g of dimethylformamide, stir well, add 100ml of dichloromethane, stir rapidly, slowly pour in 20.08g of aminothioxime acetic acid, and stir to obtain aminothiazide Clear solution of oxime acetic acid active.
[0058] (2) Add 35.10 g of 7-aminocefatriazine (7-ACT) into 200 ml of dichloromethane, stir well, cool, slowly add 20.14 g of tetramethylguanidine, and stir to obtain 7-ACT tetramethylguanidine salt solution.
[0059] (3) Add the activator solution of aminothioxime acetic acid into the solution of 7-ACT tetramethylguanidine salt for reaction. After adding 200ml of water, raise the temperature to 15°C, adjust the pH value to 4.0 with triethylamine, separate the water layer, add 1.50g of activated carbon and 0.55g of sodium bisulfite, decolorize for 30min, and filter. The pH value of the filtrate was adj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com