A method for testing the liquid bacterial retention capacity of an air filter/membrane for infusion
An air filter and testing method technology, applied in the field of testing, can solve the problems of inaccurate testing, inability to ensure safety, and poor operability, and achieve the effects of ensuring safety, reducing test errors, and simple and convenient testing operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 Determine the test pressure value:
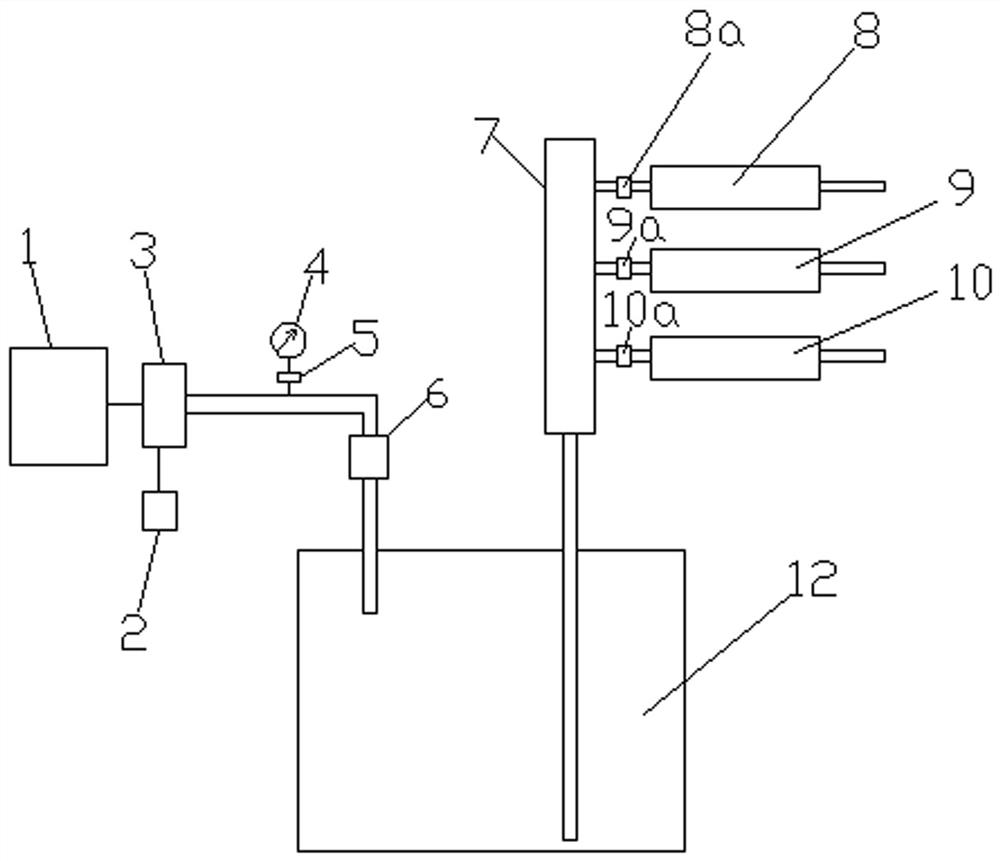
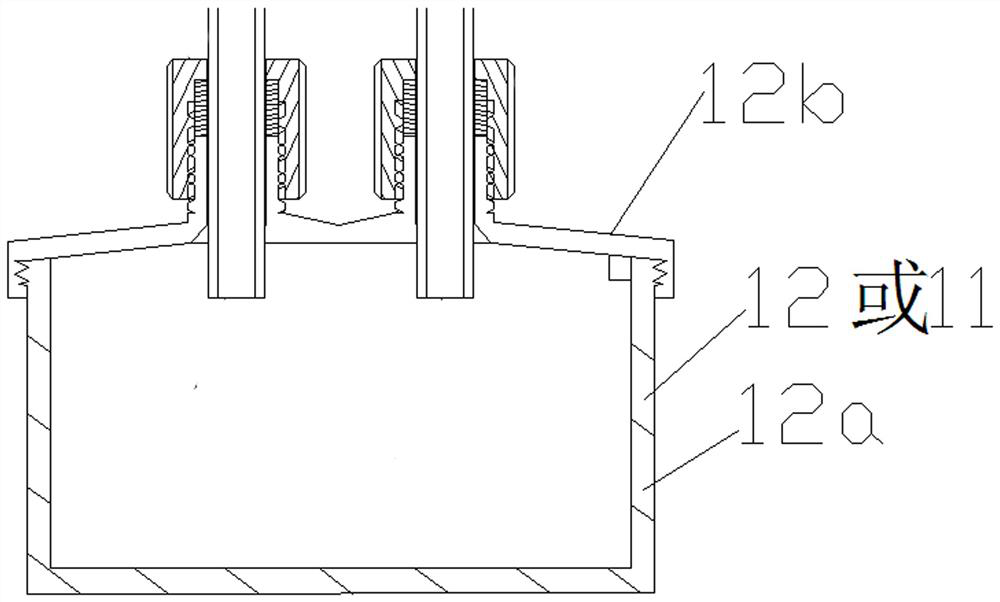
[0052] To test the air filter, challenge the direction from the inside to the outside. Such as figure 1 , Figure 4 Shown: The following test operations all took place in biological safety cabinets or ultra-clean workbenches. Unscrew the cover 12b from the bacteria tank 12, inject the challenge bacteria solution into the tank 12a, and tighten the cover 12b. Install the air filter to be tested to the test assembly, and adjust it to the state to be tested, and install the infrared liquid level probe 8c to the corresponding position of the test assembly. Plug in the power plug 13, turn on the power switch 16, and the power indicator light 15 is turned on. Turn on the negative process switch 20, the working indicator light 8b, the negative test channel solenoid valve 8a, the system solenoid valve 19a, and the negative infrared liquid level probe 8c are all automatically turned on, and then the air pump 3 is turned on, an...
Embodiment 2
[0055] Example 2 To test the air filter, the challenge direction is from inside to outside.
[0056] Such as figure 1 , Figure 4 Shown: The following test operations all took place in biological safety cabinets or ultra-clean workbenches. Connect negative test assembly 8, positive test assembly 9, and sample test assembly 10 to the detection device in the following order: control system 1, primary air filter 2, air pump 3, pressure gauge 4, pressure regulator 5, secondary air Filter 6, bacteria tank 12, diverter 7, test assembly. Plug in the power plug 13, turn on the power switch 16, and the power indicator light 15 is turned on.
[0057] Test the negative control sample: unscrew the cover 12b from the bacteria tank 12, pour sterile water into the tank 12a, and tighten the cover 12b. Install the negative control to the negative test component 8, and adjust it to the state to be tested, and install the negative infrared liquid level probe 8c to the corresponding position ...
Embodiment 3
[0060] Example 3 To test the air filter, the challenge direction is from outside to inside.
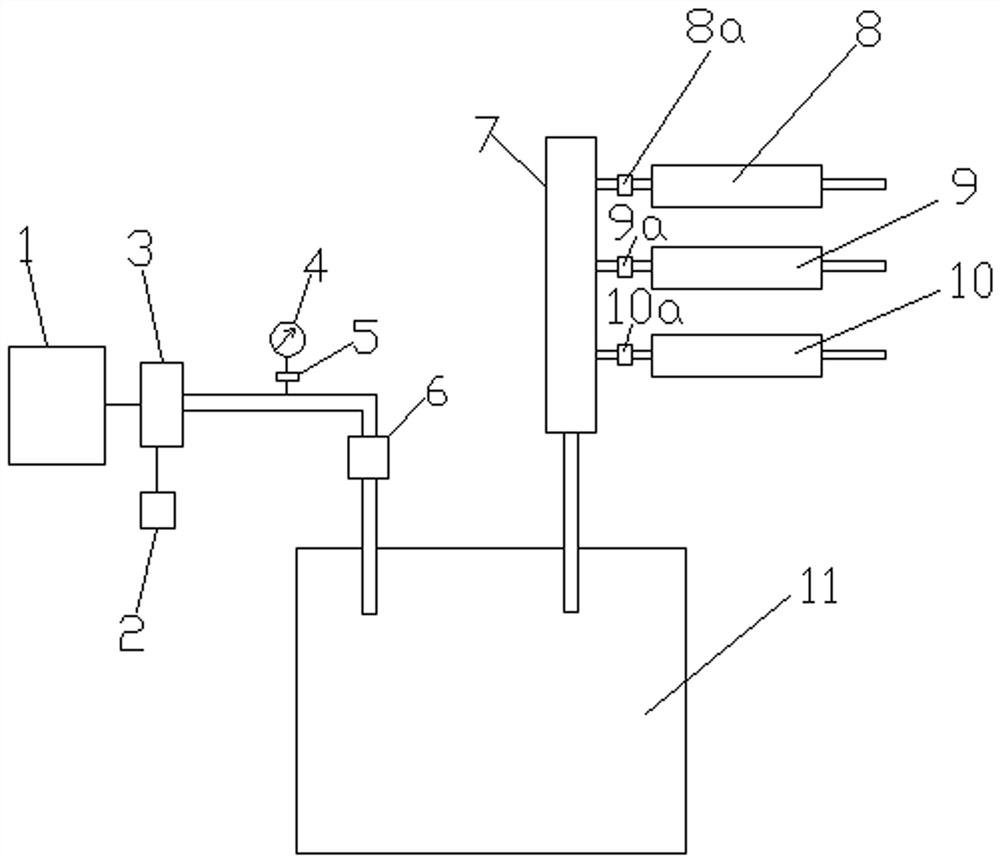
[0061] Such as figure 2 , Figure 4 Shown: The following test operations all took place in biological safety cabinets or ultra-clean workbenches.
[0062] The following test operations all took place in biological safety cabinets or ultra-clean workbenches. Connect negative test assembly 8, positive test assembly 9, and sample test assembly 10 to the detection device in the following order: control system 1, primary air filter 2, air pump 3, pressure gauge 4, pressure regulator 5, secondary air Filter 6, constant pressure tank 11, splitter 7, test assembly. Plug in the power plug 13, turn on the power switch 16, and the power indicator light 15 is turned on.
[0063] Install the negative control to the negative test component 8, inject a specific volume of sterile water into the negative test component 8; install the positive control sample (liquid) to the positive test component...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com