Benzozaphosphorophosphine oxygen ligand and its complex, preparation method and application
A technology of benzazepine phosphorus and phosphine oxide, which is applied in the field of metal catalysts and can solve the problems of low yield of carbon-carbon bond coupling and many by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
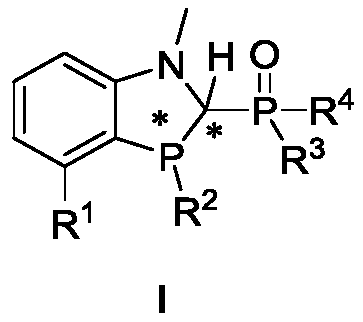
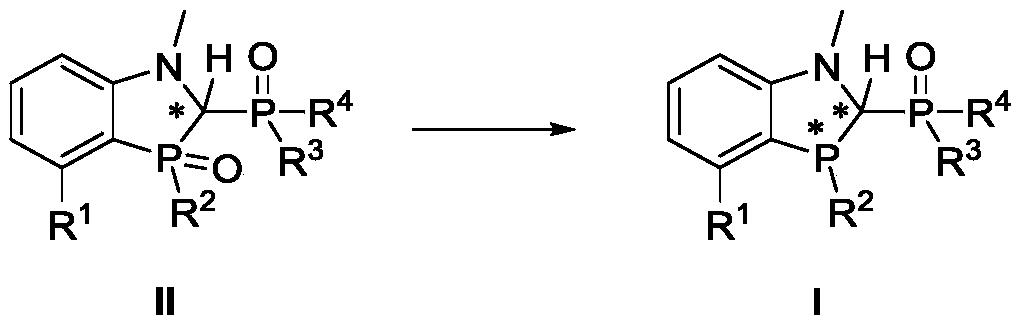
[0135]The preparation of ligand 1, the reaction scheme is as follows:
[0136]
[0137] 1. Synthesis of compound b
[0138] Take a 3L clean three-neck flask, add sulfuric acid (3×210mL, 3M), formaldehyde (125mL, 1.624mol, 4equiv) and tetrahydrofuran (700mL), then cool the above mixed system to below -20°C, and compound a (50g, 406mmol, 1.0equiv) and sodium borohydride (90g, 2.367mol, 5.8equiv) were dissolved in tetrahydrofuran and placed in a 1L clean three-neck flask, and then slowly added sulfuric acid, formaldehyde and Tetrahydrofuran mixed in the system and keep the system temperature below 0°C. In this process, sulfuric acid will be added in batches (half add at the beginning, and the remainder adds in batches during the dropwise addition). After the raw materials were added, the above system was returned to room temperature, and stirring was continued for 2 hours. TLC plate monitoring, and then the system was adjusted to strong alkalinity with potassium hydroxide a...
Embodiment 2
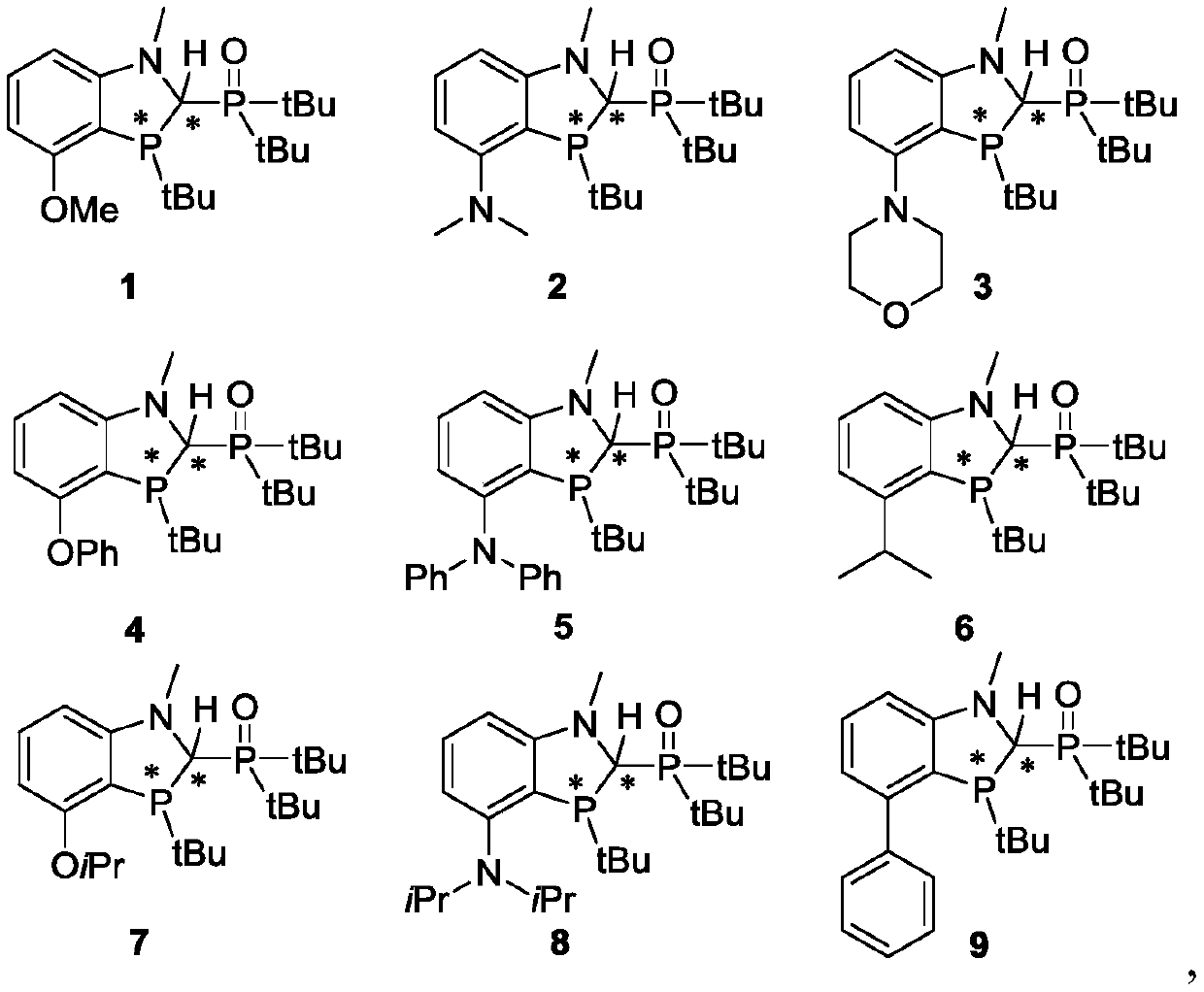
[0153] Ligand 1 Catalysis of Different Substrates
[0154] Take a 10mL Schlenk tube and dry it, add compound A (0.10mmol, 1.0equiv), tert-butylmagnesium chloride (0.40mmol, 4.0equiv), 1.5 hydrated nickel(II) chloride (2.5%mmol, 0.025equiv) and Ligand 1 (2.5% mmol, 0.025 equiv, nickel / ligand molar ratio is 1 / 1), pumped nitrogen three times, and sealed. Tetrahydrofuran (1 mL) was added. Cool the reaction system to -10°C for 1.5 hours, cool to room temperature, add water (10mL) and dichloromethane (10mL), separate the layers, combine the organic phases, dry over anhydrous sodium sulfate, concentrate, and use HPLC after sampling The reaction yield was detected by chromatography, and a colorless oily product was obtained by column chromatography, and the ratio of product C to by-product D was analyzed by NMR data.
[0155] Table 1
[0156]
[0157]
[0158] [a] Reaction conditions: nitrogen environment, tetrahydrofuran as solvent, add 2.5mol% 1.5 hydrated nickel(II) chlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com