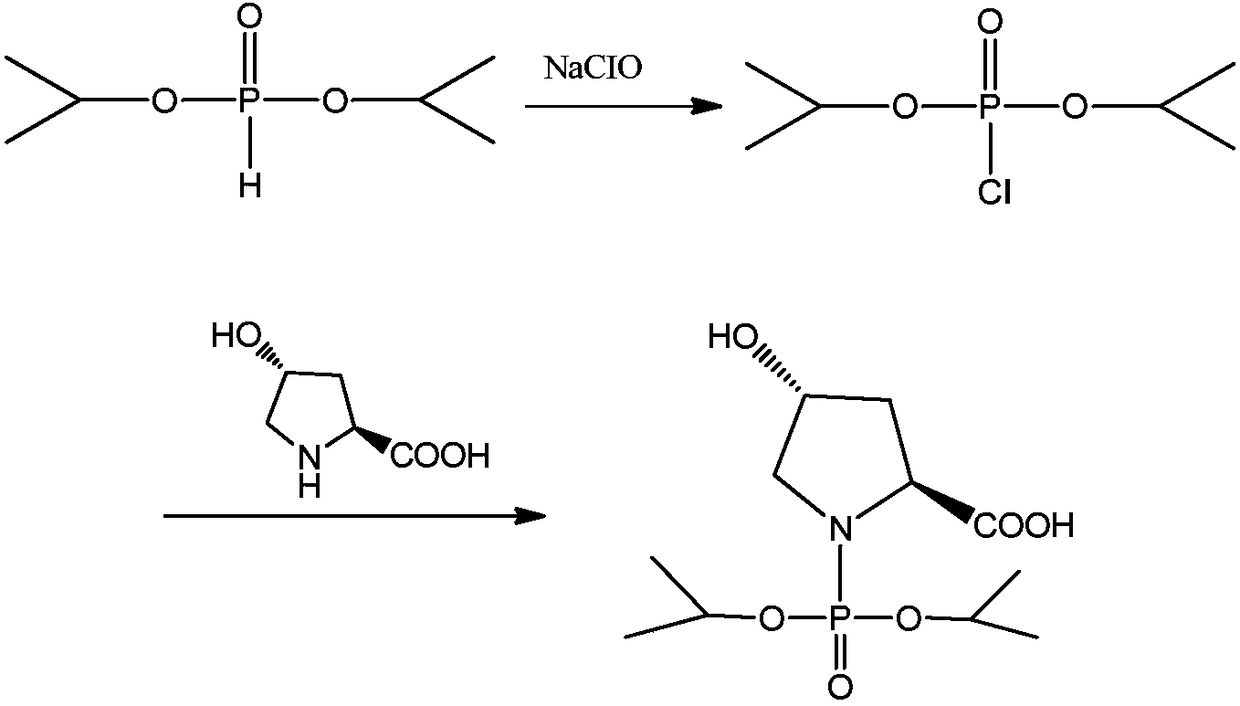
Preparation method of N-(O, O-diisopropylphosphoryl)-trans-4-hydroxy-L-proline
A technology of hydroxyproline and propylphosphoryl, which is applied in the field of pharmaceutical intermediates, can solve the problems of low product yield, waste gas generation, and many side reactions, and achieve the effects of high purity, high yield, and avoiding side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 174g (1.05mol) of DIPP into the 1000ml reaction flask, stir and cool down to -10°C, at this temperature, start to slowly add 455g (1.6mol) of sodium hypochlorite solution with an available chlorine content of 12.5%, and the dropping time is 60min. After the dropwise addition, continue to stir for 60 minutes at a speed of 400 r / min or more, and monitor the reaction by gas chromatography (IPC-1: DIPP<1.0%). After the reaction finishes, add sodium bisulfite 5g, measure and change color with starch-KI test paper.
[0035] Control the internal temperature at 5°C, add 100 g (0.915 mol) of L-Hyp in batches, finish adding 60 minutes, add 7.7 g of L-Hyp every 5 minutes, use 25% sodium hydroxide solution to maintain pH = 9.0 during the addition, and continue stirring for 45 minutes after adding. minute. When the pH no longer decreased, the dropwise addition was stopped, and the reaction was incubated for 30 minutes, and the reaction result was monitored by HPLC (IPC-2).
[...
Embodiment 2
[0038] Add 208g of pure water and 100g (0.915mol) of L-Hyp into a 500ml reaction bottle, cool down to 5°C, and start to add about 40.6g (0.254mol) of 25% sodium hydroxide solution dropwise under stirring. At this time, the pH=9.2, and the reaction solution is clear ,stand-by.
[0039] Add 174g (1.05mol) of DIPP into a 2000ml reaction flask, stir and cool down to -10°C, at this temperature, start to slowly add 455g (1.6mol) of sodium hypochlorite solution with an available chlorine content of 12.5%, and the dropping time is 60min. After the dropwise addition, continue to stir for 60 minutes at a speed of more than 400 r / min. The reaction was monitored by gas chromatography (IPC-1: DIPP<1.0%). After the reaction finishes, add sodium bisulfite 5g, measure and change color with starch-KI test paper.
[0040] Control the internal temperature at 0°C, add the L-Hyp aqueous solution to be used dropwise, and finish dropping after 60 minutes. During the addition, use 25% sodium hydrox...
Embodiment 3
[0043] Add 208g of pure water and 100g (0.915mol) of L-Hyp into the 2000ml reaction flask, cool down to 5°C, and start to add about 40.6g (0.254mol) of 25% sodium hydroxide solution dropwise under stirring, at this time, the pH=9.3, and the reaction solution clarify. stand-by.
[0044]Add 174g (1.05mol) of DIPP into the 1000ml reaction flask, stir and cool down to -10°C, at this temperature, start to slowly add 455g (1.6mol) of sodium hypochlorite solution with an available chlorine content of 12.5%, and the dropping time is 60min. After the dropwise addition was completed, stirring was continued for 60 minutes, and the reaction was monitored by gas chromatography (IPC-1: DIPP<1.0%). After the reaction finishes, add sodium bisulfite 5g, measure and change color with starch-KI test paper, as diisopropylphosphorous oxychloride reaction liquid.
[0045] The diisopropylphosphorous oxychloride reaction solution was kept stirring, cooled to -5°C, and the diisopropyl phosphorous ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com