Novel crystalline form of chlorfenapyr, process for its preparation and use
A technology of chlorfenapyr and crystallization, which is applied in the field of agricultural chemical preparations to achieve the effects of improved storage stability, low aggregation tendency and long storage period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1: Preparation of amorphous chlorfenapyr according to the disclosure of US Patent No. 5,359,090 Example 1
[0121] in N 2 Next, 4-bromo-2-(4-chlorophenyl)-5-(trifluoromethyl)pyrrole-3-carbonitrile (17.4g, 0.05 mole), diethoxymethane (10.4g, 0.10 mole ) and dimethylformamide (DMF) (4.6 g, 0.0625 mol) in toluene were treated batchwise with phosphorus oxychloride (9.6 g, 0.0625 mol) at 35°C to 45°C over a period of 10 minutes Work up by heating at 45°C to 53°C for about 0.5 hours, cooling to 35°C and treating with triethylamine (7.25 g, 0.0715 mol) dropwise over 2 hours at 35°C to 45°C. The reaction mixture was treated with water, and the toluene was removed via azeotropic distillation. The remaining residue was treated with water, filtered and the filter cake was dried under vacuum at 60 °C to give the title product 20.8 g, 92.7% purity, 94.6% yield, identified by HPLC analysis.
[0122]
[0123] Scheme 1. Synthesis of Chlorfenapyr
[0124] Such as image ...
Embodiment 2
[0125] Example 2: Preparation of chlorfenapyr crystalline modification I
[0126] Crystallized from methyl ethyl ketone
[0127] 10 g of the amorphous chlorfenapyr sample prepared in Example 1 was put into a three-necked round bottom flask together with 50 ml of methyl ethyl ketone, and the resulting slurry was heated to 65° C. to obtain a homogeneous solution. Undissolved particles (if any) were filtered and the solution was slowly cooled to 20°C to 25°C. Upon cooling, fine crystals formed and the resulting heterogeneous mixture was stirred at 20 °C for 2 h. Then, the slurry was filtered and washed with 3 ml of methyl ethyl ketone (20°C). The filtered crystals were dried under vacuum at 40°C. The purity of the obtained crystalline product was >98%, and the yield was found to be not lower than 90%.
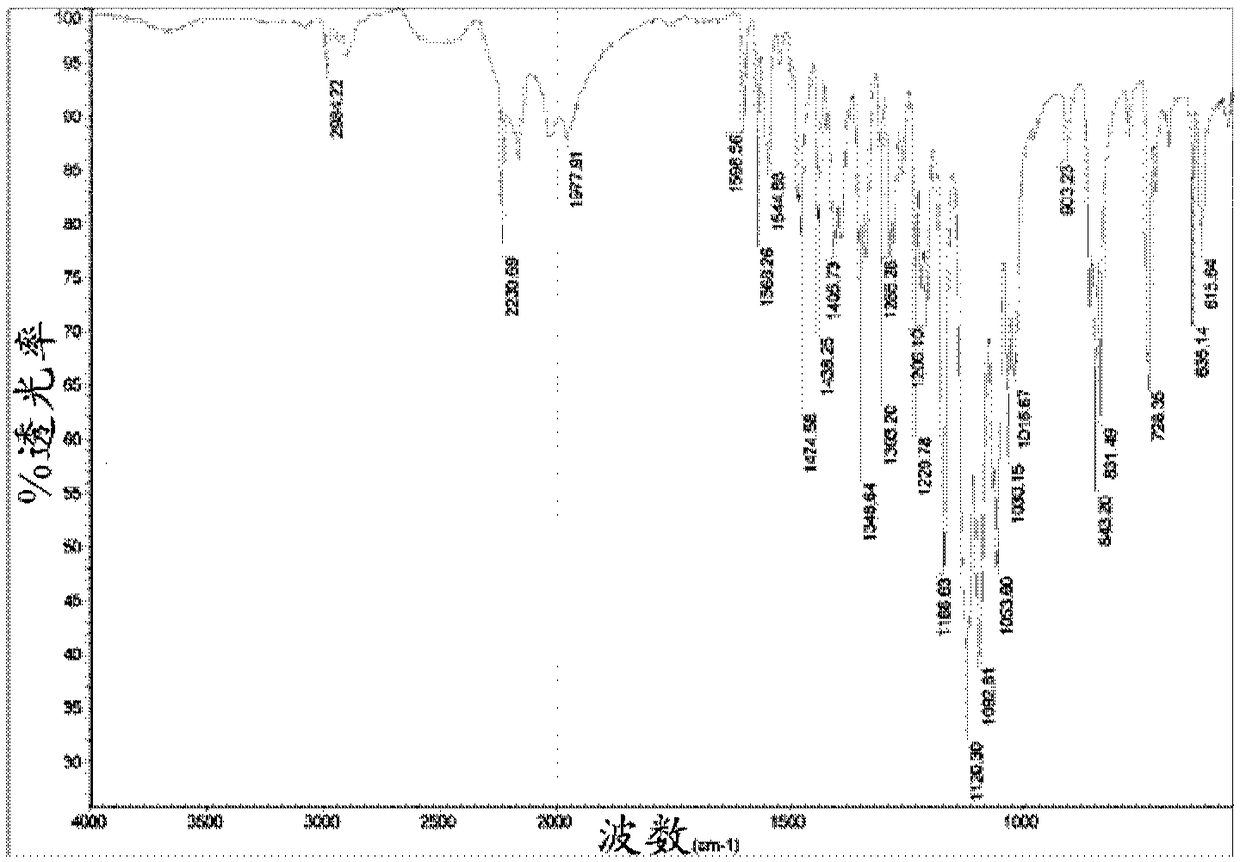
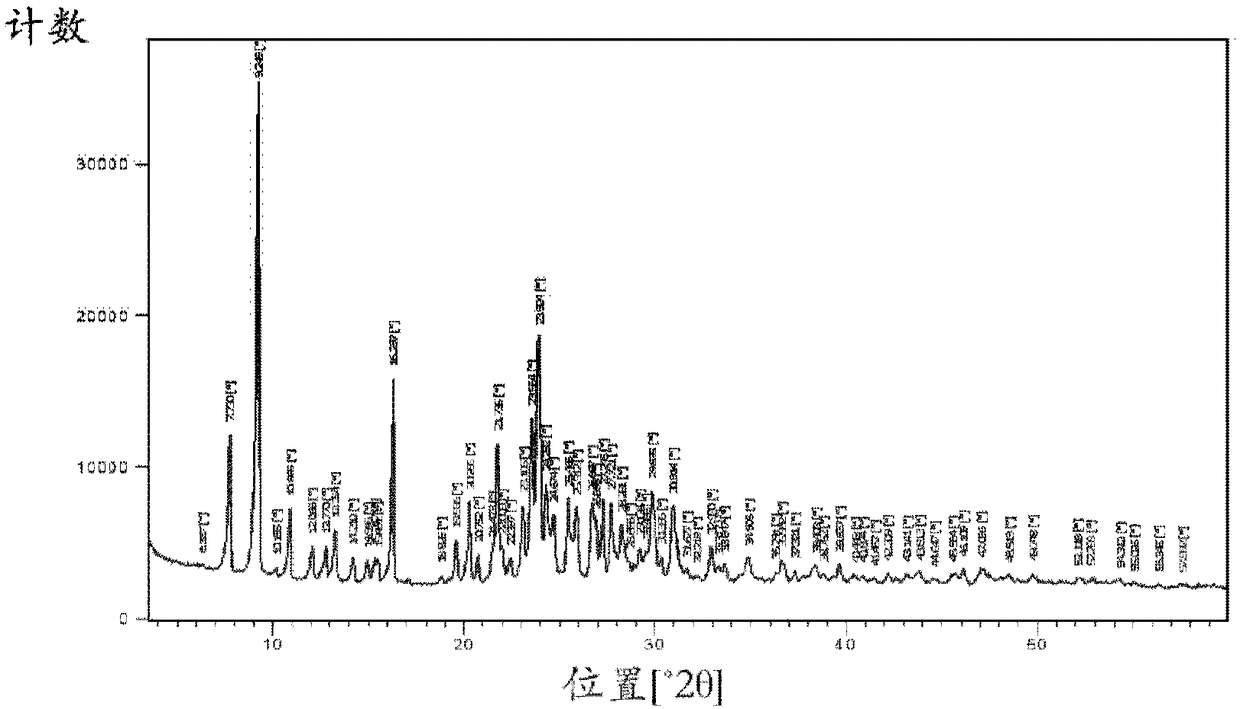
[0128] The obtained crystals were analyzed by IR spectroscopy and X-ray powder diffraction, and found to be as figure 1 and figure 2 Chlorfenapyr crystalline modification...
Embodiment 3
[0133] Example 3: Preparation of chlorfenapyr crystalline modification I
[0134] Crystallized from nitrobenzene
[0135] 5 g of the amorphous chlorfenapyr sample prepared in Example 1 was put into a three-necked round bottom flask together with 30 ml of nitrobenzene, and the resulting slurry was heated to 83° C. to obtain a homogeneous solution. Undissolved particles (if any) were filtered and the solution was slowly cooled to 20°C to 25°C. Upon cooling, fine crystals formed and the resulting heterogeneous mixture was stirred at 20 °C for 2 h. Then, the slurry was filtered and washed with 3 mL of nitrobenzene. The filtered crystals were dried under vacuum at 45°C. The purity of the obtained crystalline product was >98%, and the yield was found to be not lower than 90%.
[0136] These crystals were characterized as chlorfenapyr crystalline modification I using IR spectroscopy, X-ray powder diffraction and DSC as described in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com