System and method for protein corona sensor array for early detection of diseases
A technology of sensor array and protein, applied in the field of sensor array
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0314] Example 1A is used for cancer early detection Label-free sensor array
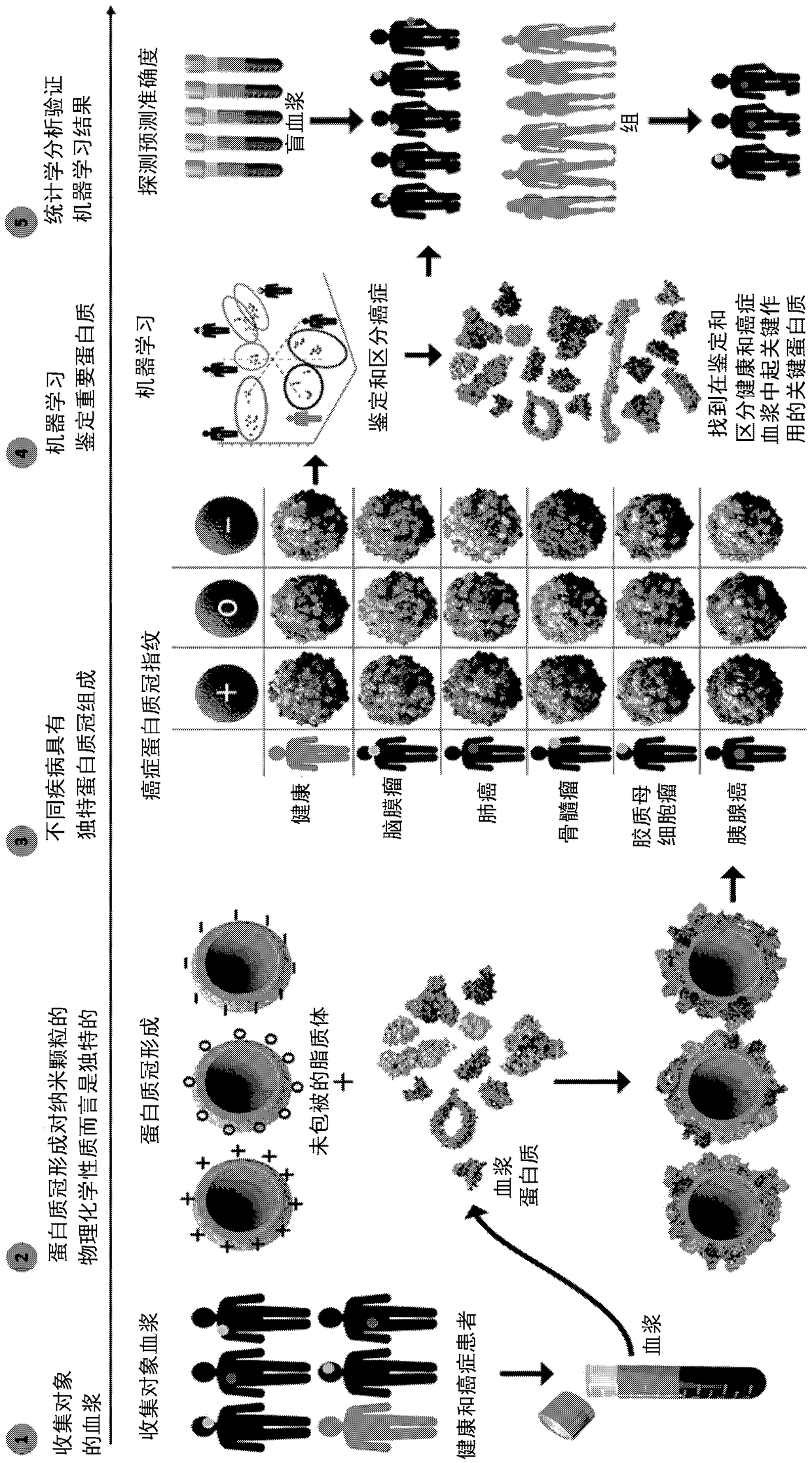
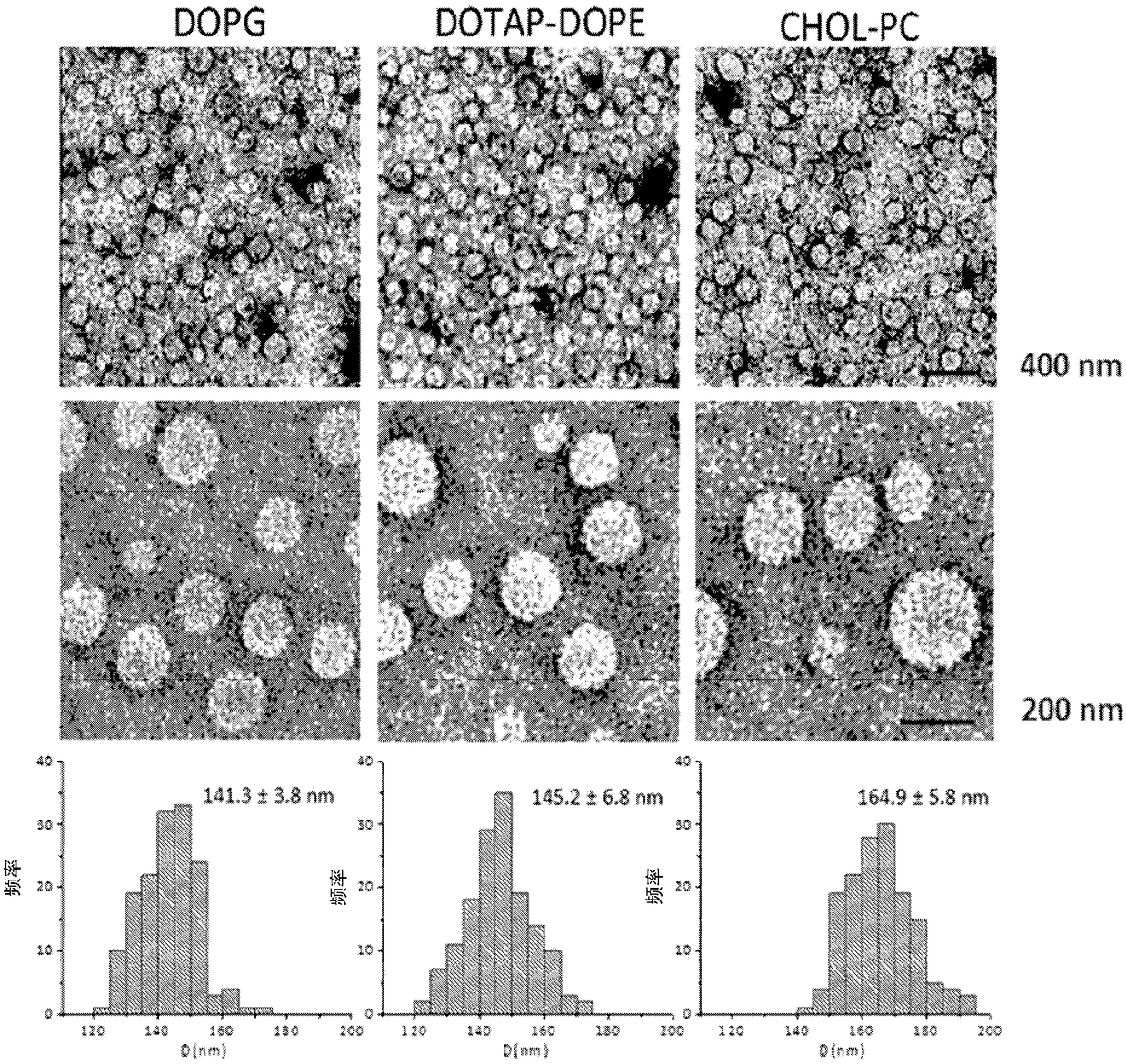
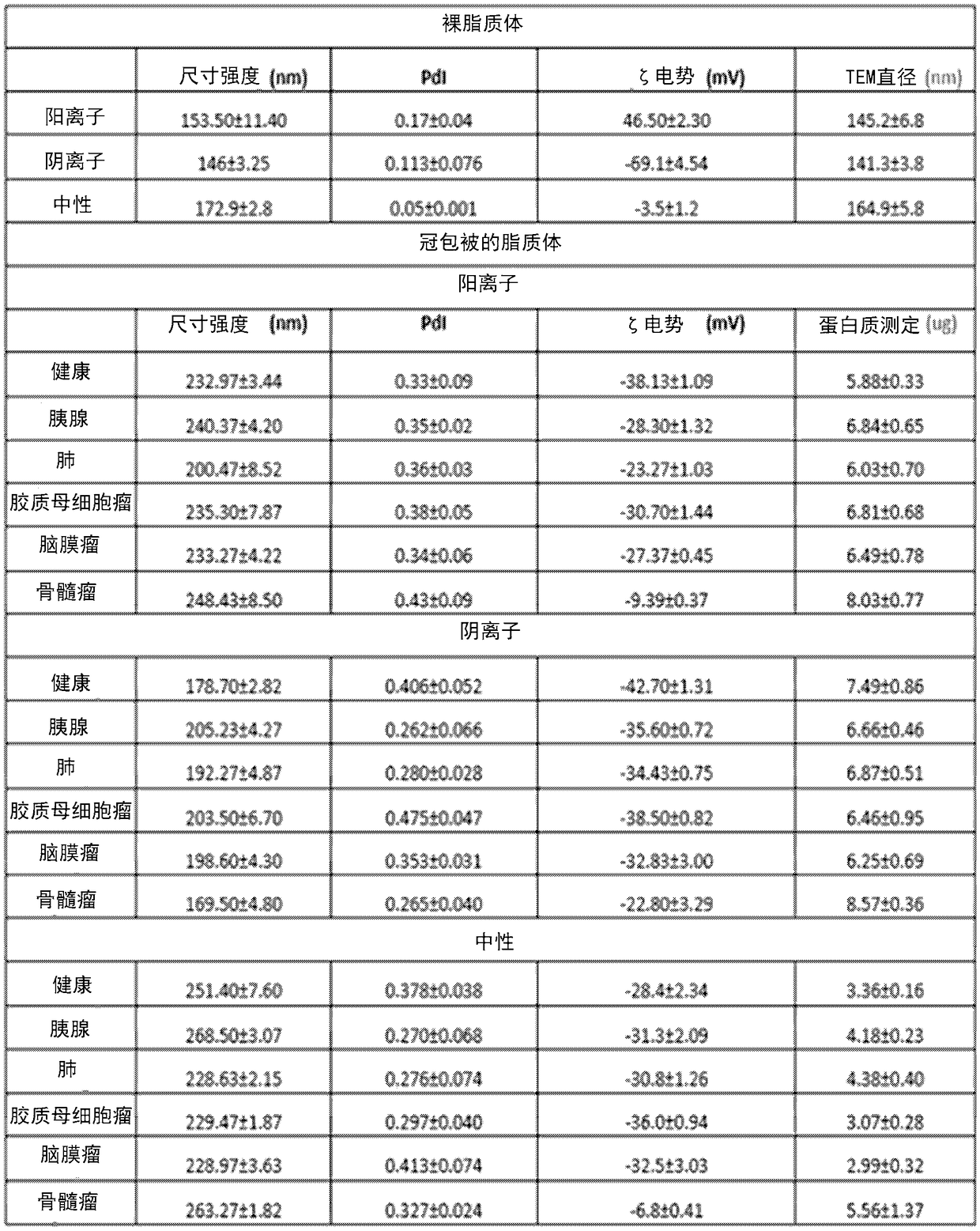
[0315]This example provides a label-free sensor array for early detection of various cancers. The sensor array consists of three different cross-reactive liposomes with different surface charges (i.e., cationic (DOPG(1,2-dioleoyl-sn-glycero-3-phosphate-(1′-rac-glycerol)), anionic (DOTAP (1,2-dioleoyl-3-trimethylammonium-propane)-DOPE (dioleoylphosphatidylethanolamine) and neutral (CHOL (DOPC-cholesterol)), whose protein corona composition increases with their association with The interaction of plasma from patients with different types of cancer, namely lung cancer, pancreatic cancer, myeloma, meningioma and glioblastoma varied. Although no single protein corona composition is specific for any one cancer type, the corona Changes in compositional patterns provide each type of cancer with a unique "fingerprint".
[0316] Hard crown profiling of sensor array elements using plasma from early, inte...
Embodiment 1B
[0361] Example 1B Deep profiling and machine learning of the human proteome using multinanoparticle protein corona characterization enables Accurately identify and differentiate cancer at an early stage
[0362] In a second embodiment, collective protein corona data for a given plasma sample from protein corona profiles of individual nanoparticles can be analyzed using machine learning (eg, random forest methods) to identify and differentiate different types of cancers. The collected data was analyzed in further detail using the sensor array as described in Example 1A and by machine learning. This sensor array (ie protein corona nanosystem) clearly and robustly identifies cancer and allows different types of cancer to be differentiated. This system can be used to predict cancer type using blinded plasma samples. The ability to use plasma from healthy people for very early detection in existing cohorts diagnosed with cancer years after plasma collection to determine a prec...
Embodiment 2
[0425] Example 2. Additional sensor array configurations for detection of disease
[0426] A sensor array composed of 12 different cross-reactive nanoparticles was prepared, including three kinds of liposomes, three kinds of superparamagnetic iron oxide nanoparticles and six kinds of gold nanoparticles. These types of liposomes (DOPG (1,2-dioleoyl-sn-glycero-3-phosphate-(1′-rac-glycerol)) with negative, neutral and positive surface charges were synthesized according to our previous report, DOTAP (1,2-dioleoyl-3-trimethylammonium-propane)-DOPE (dioleoylphosphatidylethanolamine), and CHOL (DOPC-cholesterol)). From Ultra-uniform PEG-coated superparamagnetic iron oxide nanoparticles of 20 nm size and various PEG molecular weights (ie 300, 3000 and 6000) were obtained. Gold nanoparticles with a core size of about 2 nm and different surface functionalities were synthesized.
[0427] All nanoparticles have the same size but different surface properties, which are expected to fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com