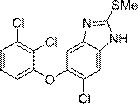
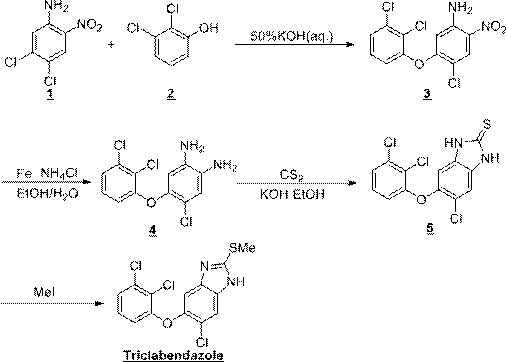
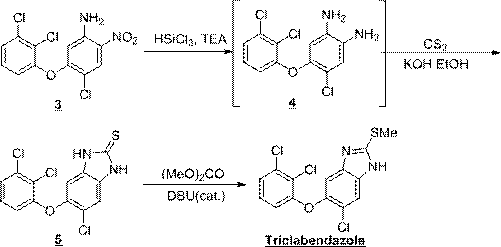
Synthesis method of triclabendazole
A technology of triclbendazole and a synthetic method, applied in directions such as organic chemistry, can solve problems such as unmentioned substances and product purity, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] intermediate 5 Preparation of:
[0049] 4-Chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline (50 g, 0.15 mol) and triethylamine (75.8 g, 0.75 mol) were dissolved in dichloromethane (300 ml). Under the condition of nitrogen protection, cool to 0~10°C, add dropwise a solution of trichlorosilane (71.1g, 0.53mol) in dichloromethane (100ml), keep the temperature not higher than 10°C during the dropping process; after the dropwise addition, React at 15~20°C for 12 hours; quench the reaction with saturated sodium bicarbonate solution, adjust the pH to 8~9, stir for 0.5 hours; let stand to separate the liquid, wash with water to obtain the intermediate 5 dichloromethane solution.
[0050] Add ethanol (500ml) to the above solution, distill at normal pressure until the boiling point of the distillate is above 78°C (ethanol needs to be added in the middle, the final volume is about 500ml), and the intermediate is obtained 5 weak. In another reaction flask, dissolve potassium hydrox...
Embodiment 2
[0055] intermediate 5 Preparation of:
[0056] 4-Chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline (50g, 0.15mol) and triethylamine (60.6g, 0.60mol) were dissolved in dichloromethane (300ml). Under the condition of nitrogen protection, cool to 0~10°C, add dropwise a solution of trichlorosilane (80.5g, 0.60mol) in dichloromethane (100ml), keep the temperature not higher than 10°C during the dropping process; after the dropwise addition, React at 15~20°C for 12h; quench the reaction with saturated sodium bicarbonate solution, adjust the pH to 8~9, stir for 0.5h; let stand to separate the liquid, wash with water to obtain the intermediate 5 dichloromethane solution.
[0057] Add ethanol (500ml) to the above solution, distill at normal pressure until the boiling point of the distillate is above 78°C (ethanol needs to be added in the middle, the final volume is about 500ml), and the intermediate is obtained 5 weak. In another reaction bottle, dissolve potassium hydroxide (9.8g, 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com