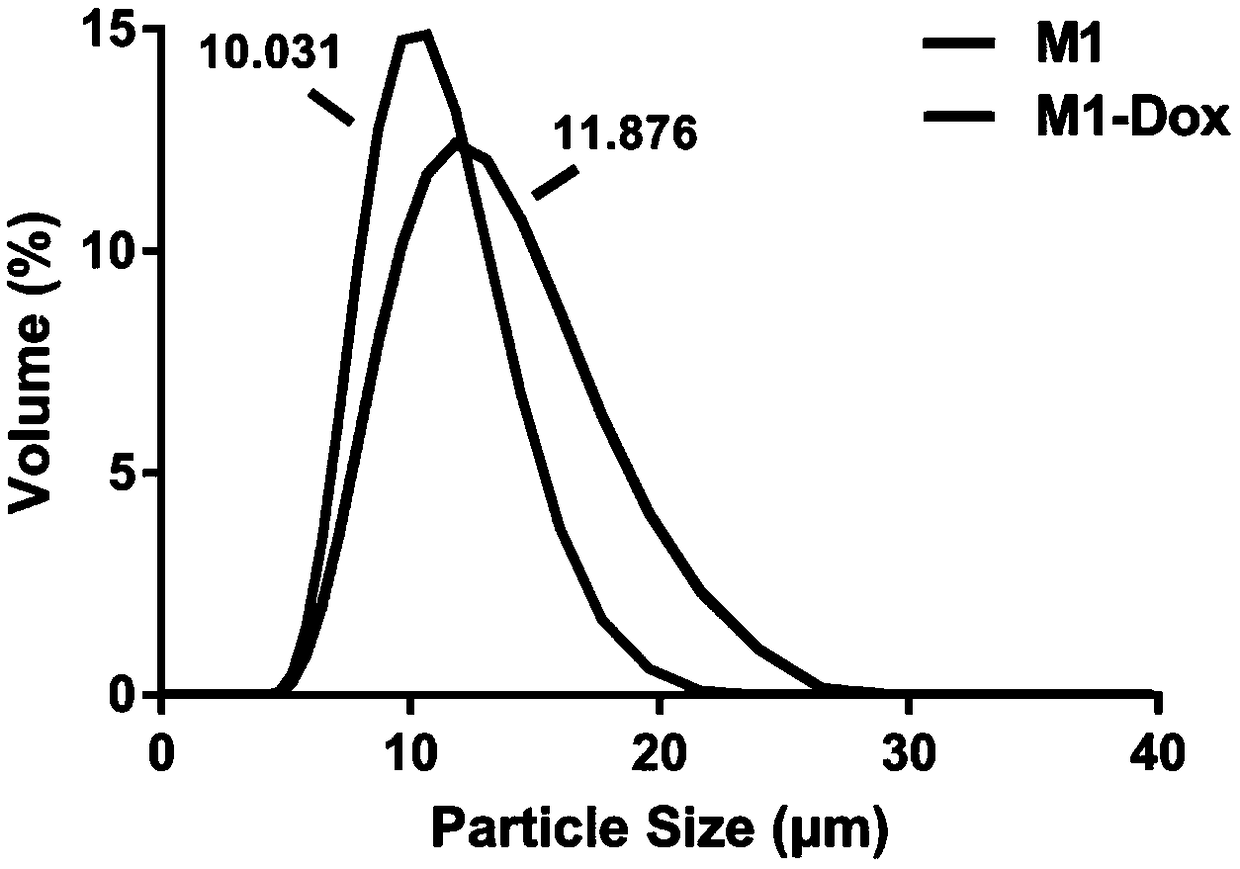
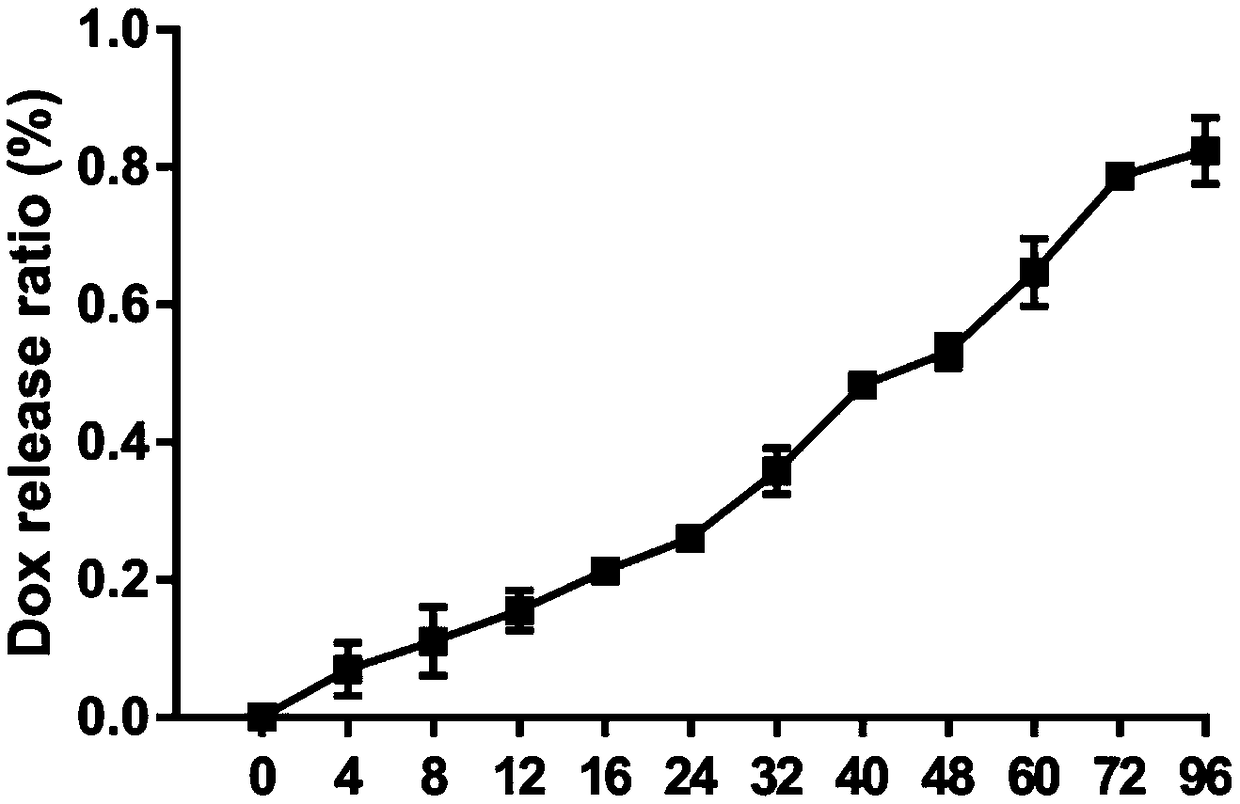
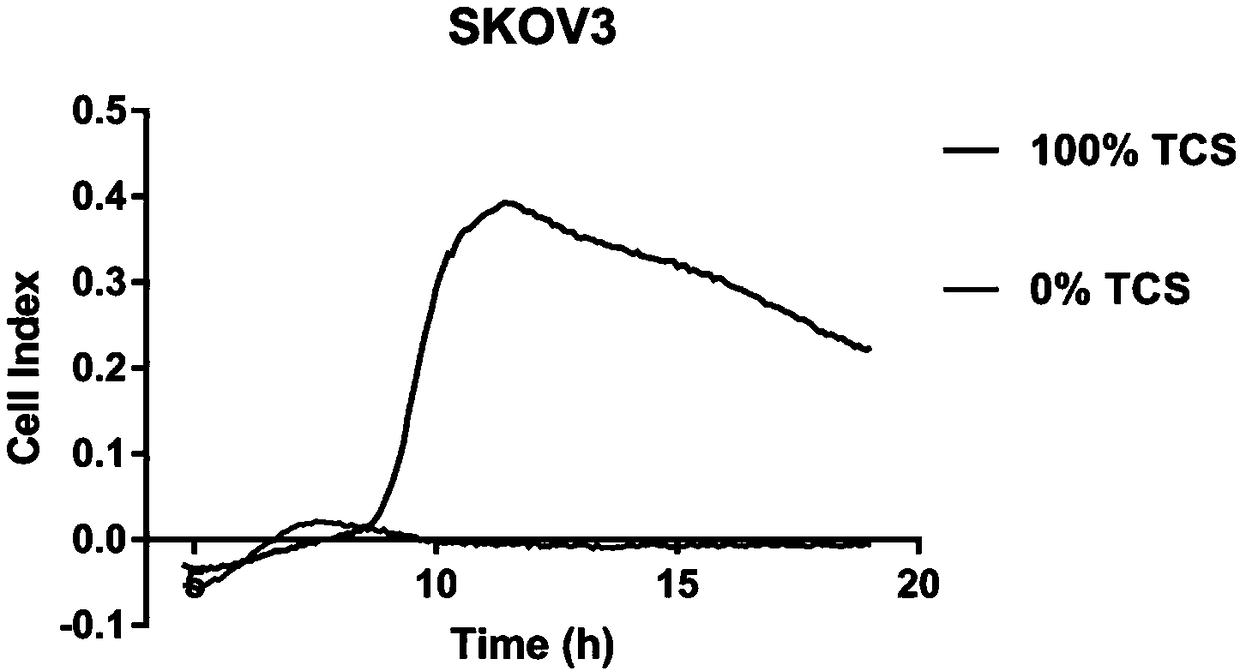
Macrophage drug-loading preparation and preparation method thereof
A macrophage and drug-loading technology, which is applied in pharmaceutical formulations, anti-tumor drugs, drug combinations, etc., to achieve good anti-tumor activity and good tumor suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of drug-loaded preparations of M1 macrophages loaded with doxorubicin
[0033] Preparation of fat-soluble doxorubicin:
[0034] (1) Weigh 20 mg of doxorubicin hydrochloride (purchased from Dalian Meilun Biotechnology Co., Ltd.) into a 100 ml round bottom flask, and add 20 ml of dichloromethane to fully dissolve it.
[0035] (2) Add 14.3mL of triethylamine according to the molar ratio of 1:3, and stir overnight at 25°C;
[0036] (3) Collect the product after 24 hours and place it in a dialysis bag for dialysis until the dialysate is clear and colorless;
[0037] (4) Collect the material in the dialysis bag and freeze-dry it to obtain dark red doxorubicin powder;
[0038] (5) Dissolve 1.630 mg of doxorubicin powder in 1 mL of DMSO to make a final concentration of 3 mmol / L.
[0039] Preparation of M1 macrophages:
[0040] (1) RAW264.7 cells (purchased from the Animal Experiment Center of Sun Yat-Sen University) were placed in high-glucose DMEM me...
Embodiment 2
[0046] Example 2: Preparation of drug-loaded preparations of M1 macrophages loaded with doxorubicin
[0047] Preparation of fat-soluble doxorubicin:
[0048] (1) Weigh 20mg of doxorubicin hydrochloride in a 100ml round bottom flask, add 20mL of dichloromethane to fully dissolve.
[0049] (2) Add 14.3mL of triethylamine according to the molar ratio of 1:3, and stir overnight at 25°C;
[0050] (3) Collect the product after 24 hours and place it in a dialysis bag for dialysis until the dialysate is clear and colorless;
[0051] (4) Collect the material in the dialysis bag and freeze-dry it to obtain dark red doxorubicin powder;
[0052] (5) Dissolve 2.445 mg of doxorubicin powder in 1 ml of DMSO to make a final concentration of 4.5 mmol / L.
[0053] Preparation of M1 macrophages:
[0054] (1) RAW264.7 cells were cultured in high-glucose DMEM medium containing 10% fetal bovine serum at 37°C and 5% CO 2 Subculture under the culture conditions.
[0055] (2) After 24 hours of pass...
Embodiment 3
[0060] Example 3: Preparation of drug-loaded preparations of M1 macrophages loaded with doxorubicin
[0061] Preparation of fat-soluble doxorubicin:
[0062] (1) Weigh 20 mg of doxorubicin hydrochloride (purchased from Dalian Meilun Biotechnology Co., Ltd.) into a 100 ml round bottom flask, and add 20 ml of dichloromethane to fully dissolve it.
[0063] (2) Add 14.3mL of triethylamine according to the molar ratio of 1:3, and stir overnight at 25°C;
[0064] (3) Collect the product after 24 hours and place it in a dialysis bag for dialysis until the dialysate is clear and colorless;
[0065] (4) Collect the material in the dialysis bag and freeze-dry it to obtain dark red doxorubicin powder;
[0066] (5) Dissolve 0.815 mg of doxorubicin powder in 1 mL of DMSO to make a final concentration of 1.5 mmol / L.
[0067] Preparation of M1 macrophages:
[0068] (1) RAW264.7 cells were cultured in high-glucose DMEM medium containing 10% fetal bovine serum at 37°C and 5% CO 2 Subcultu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com