Method for measuring oxygen penetration of hollow capsule
A hollow-capsule, oxygen-permeable technology, applied in measuring devices, color/spectral property measurement, instruments, etc., can solve the problem of not being suitable for the detection of oxygen-permeability performance of hollow capsules, and achieve a flexible and simple method to ensure activity, quality, and method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The mensuration of embodiment 1 hypromellose hollow capsule oxygen transmission rate
[0023] A method for measuring the oxygen transmission rate of hollow capsules, comprising the following steps:
[0024] Step 1, take a batch of hypromellose hollow capsules (batch number H217040N022), and randomly select 6 capsules;
[0025] Step 2: Accurately weigh six portions of 250 mg ascorbic acid, fill them into the hollow capsules taken out in step 1, place them in a drug stability test box at 40°C±2°C, 75%±5%, and take them out after 7 days;
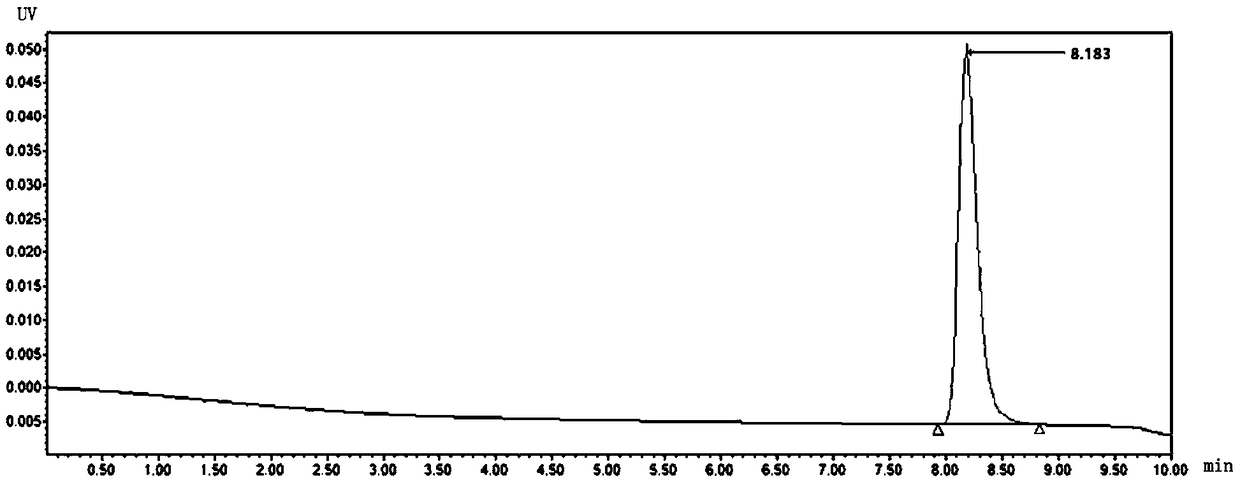
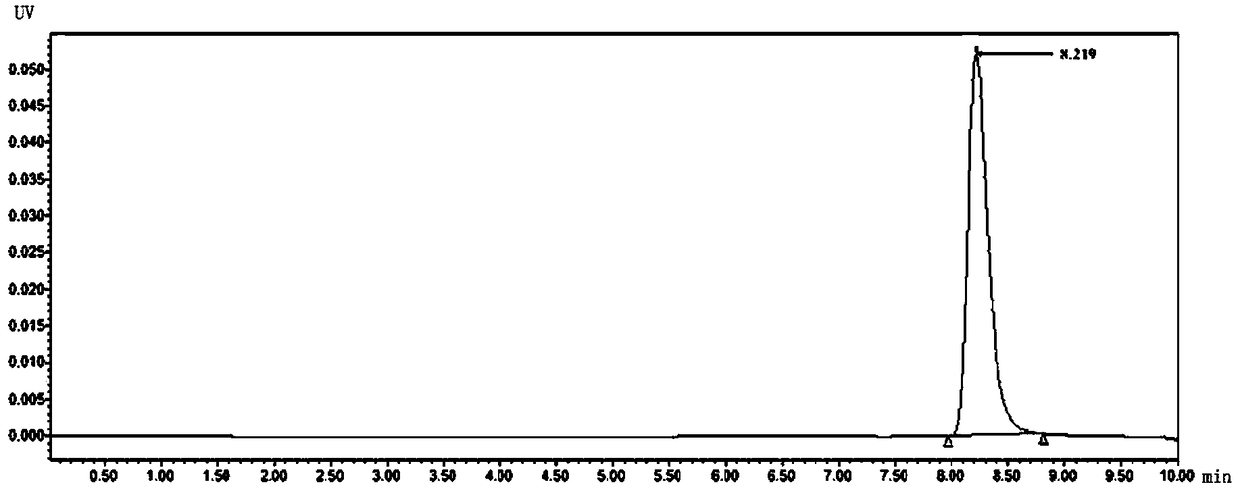
[0026] Step 3, take out two samples, open the capsule, weigh 0.050±0.0003g of the contents (accurately weighed) in a suitable container, add an appropriate amount of 0.005mol / L sulfuric acid solution to dissolve, put it in a 100ml volumetric flask, and use Dilute 0.005mol / L sulfuric acid solution to the mark, shake well; accurately measure 1ml in a 100ml volumetric flask, dilute it to 100ml with 0.005mol / L sulfuric acid solution, as the...
Embodiment 2
[0034] The method validation of embodiment 2 gelatin hollow capsule oxygen transmission rate measurement
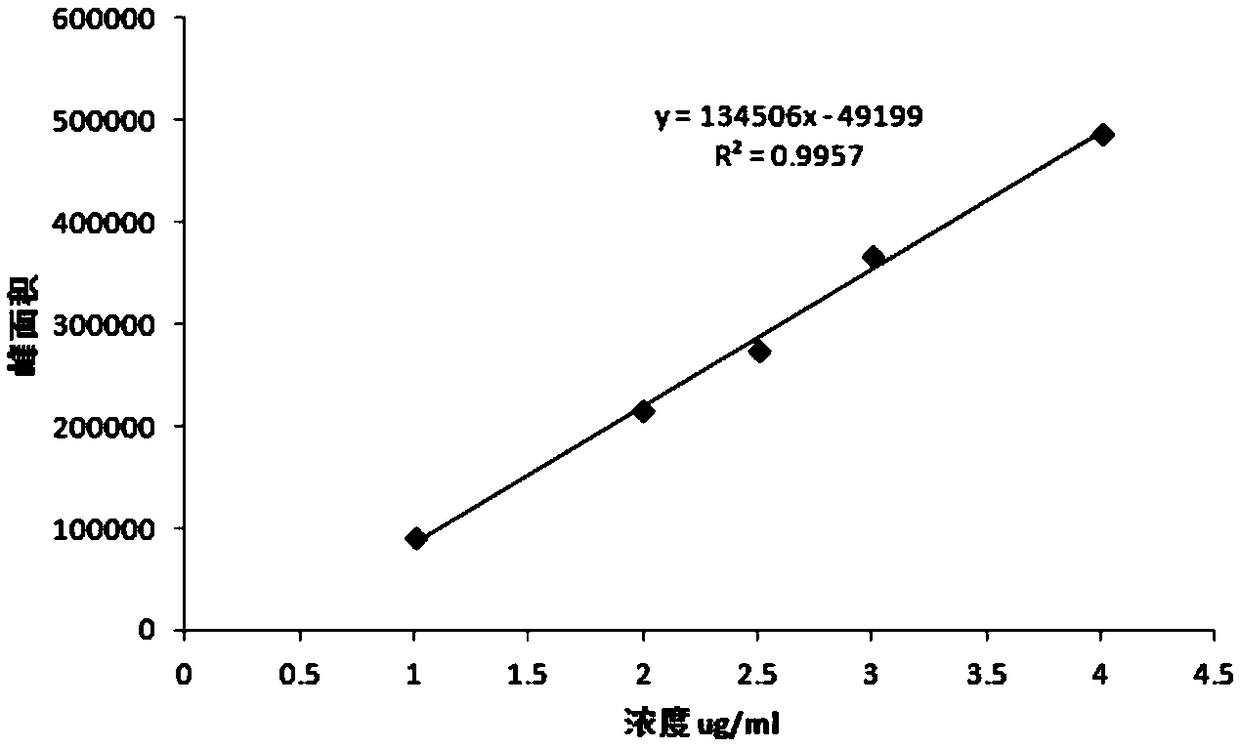
[0035] 1. Preparation of reference solution: Weigh 0.050±0.0003g of ascorbic acid (accurately weighed), put it in a suitable container, add an appropriate amount of 0.005mol / L sulfuric acid solution to dissolve it, put it in a 100ml volumetric flask, and add 0.005mol / L sulfuric acid Dilute the solution to the mark and shake well. Precisely measure 1ml into a 100ml volumetric flask, dilute to the mark with 0.005mol / L sulfuric acid solution, shake well, and you get it;
[0036] 2. Preparation of the test solution: Precisely weigh 250 mg of ascorbic acid, fill it into hollow capsules, put it in a 40°C ± 2°C, 75% ± 5% drug stability test box, and take it out after 7 days. Take the sample after 7 days of acceleration in the stability test box, open the capsule, accurately weigh the content of ascorbic acid 0.050±0.0003g, put it in a suitable container, add an appropriate amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com