Crystal form of trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2
A technology of o-chlorobenzyl pyrrolidone and crystal form, applied in the field of crystal form of trans-4-phenyl-5-o-chlorobenzyl pyrrolidone-2, achieves good reproducibility, improves bioavailability, and enhances learning effect of memory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
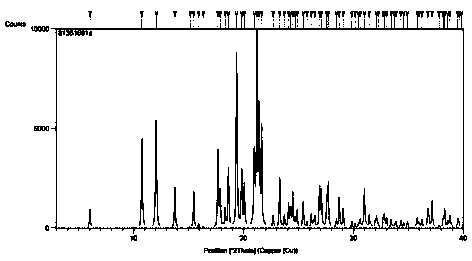
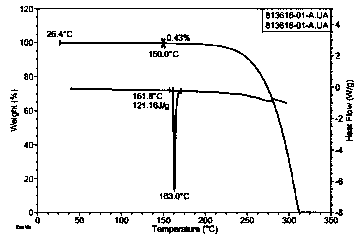
[0029] Take 100 mg trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 sample, prepare acetone / ethyl acetate (1:1) mixed solvent 50 ℃ nearly saturated solution, drop to 0 at a rate of 0.5 ℃ / min Crystallize at °C, collect the crystals, and dry to prepare a trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 crystal form A sample.
[0030] The single crystal diffraction data of the crystal were measured, and the diffraction pre-experiment and diffraction data collection were carried out using a Bruker D8VENTURE single crystal diffractometer (Mo / Kα, ) in a temperature environment of 175K. Diffraction data were processed using the APEX3 software package. Based on the 9943 diffraction points collected within the angle range of 2.383°figure 2 .
Embodiment 2
[0032] Take 200mg trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 sample, prepare methyl isobutyl ketone / 2-methyltetrahydrofuran (1:1) mixed solvent 50 ℃ nearly saturated solution, at 0.3 ℃ / min rate was reduced to 2°C for crystallization, and the crystals were collected and dried to obtain a trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 crystal form A sample.
Embodiment 3
[0034] Take 200 mg trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 sample, prepare tetrahydrofuran / n-heptane (1:1) mixed solvent 50 ℃ nearly saturated solution, drop to 5 at a rate of 0.1 ℃ / min Crystallize at °C, collect the crystals, and dry to prepare a trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 crystal form A sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com