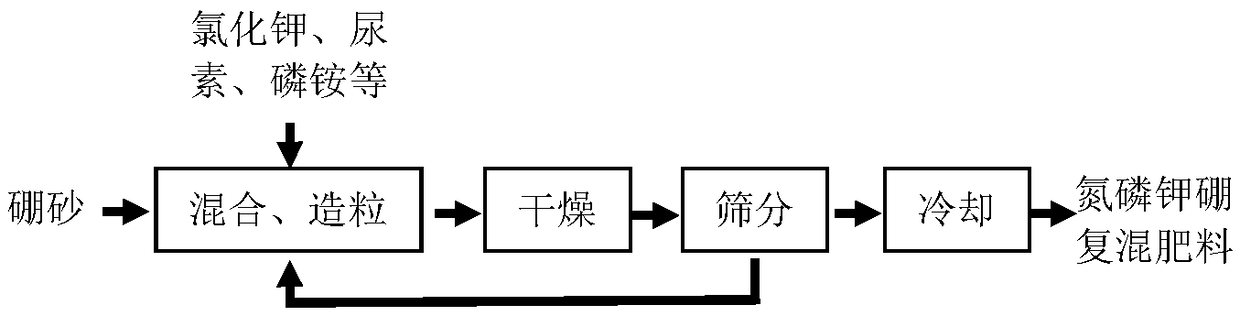
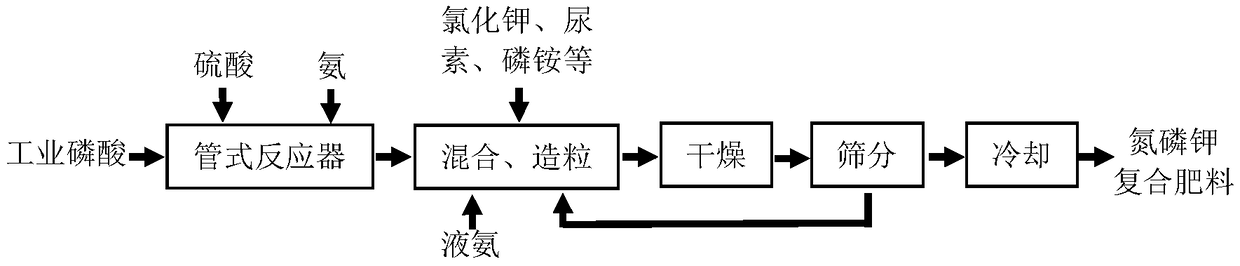
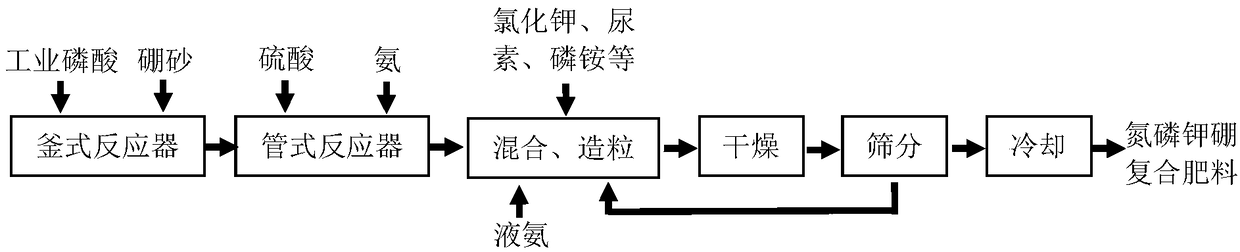
Process for preparing nitrogen-phosphorus-potassium-boron compound fertilizer
A compound fertilizer and preparation technology, which is applied in the direction of urea compound fertilizer, ammonium orthophosphate fertilizer, alkaline orthophosphate fertilizer, etc., can solve the problems that boron is difficult to be uniform, and borax or boric acid crystals are difficult to dissolve, so as to improve the solubility and prepare The method is simple and fast, and the effect of good solubility performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1, according to mass proportion, with 4.643 parts of borax (containing 0.5 part of boron of B), 246 parts of wet-process phosphoric acid (P 2 o 5 content of 93.5 parts) was added to the tank reactor; stirred at 50°C for 60 minutes, and the stirring speed was 45 rpm;
[0034] Step 2, add the materials in the tank reactor, 157 parts of sulfuric acid, and 73.61 parts of liquid ammonia (including 60.01 parts of N) into the tubular reactor and react quickly at 120°C (reaction time is about 1 second);
[0035] Step 3, with 268 parts of potassium chloride (containing K 2 O 160.8 parts), 159.6 parts of urea (containing 73.42 parts of N), 150 parts of ammonium dihydrogen phosphate (containing 16.5 parts of N, P 2 o 5 66 parts) were simultaneously added to the granulator, and the material in the tubular reactor and 12.99 parts of liquid ammonia (containing N10.59 parts) were sprayed into the granulator in an atomized state at the same time for mixing and granulation;
[...
Embodiment 2
[0038] Step 1, according to the mass proportion, 7.429 parts of borax (containing 0.8 part of B boron), 246 parts of wet-process phosphoric acid (P 2 o 5content of 93.5 parts) was added to the tank reactor; stirred at 60°C for 60 minutes, and the stirring speed was 55 rpm;
[0039] Step 2, add the material in the tank reactor, 157 parts of sulfuric acid, and 73.61 parts (including 60.01 parts of N) of liquid ammonia to the tubular reactor and react quickly at 120°C (reaction time is about 1 second);
[0040] Step 3, with 268 parts of potassium chloride (containing K 2 O 160.8 parts), 159.6 parts of urea (containing 73.42 parts of N), 150 parts of ammonium dihydrogen phosphate (containing 6.5 parts of N, P 2 o 5 66 parts) were simultaneously added to the granulator, and the material in the tubular reactor and 12.99 parts of liquid ammonia (containing N10.59 parts) were sprayed into the granulator in an atomized state at the same time for mixing and granulation;
[0041] Ste...
Embodiment 3
[0043] Step 1, according to mass proportion, with 4.643 parts of borax (containing 0.5 part of boron of B), 272 parts of wet-process phosphoric acid (P 2 o 5 content of 103.36 parts) was added to the tank reactor; stirred at 50°C for 60 minutes, and the stirring speed was 45 rpm;
[0044] Step 2, add the material in the tank reactor, 174 parts of sulfuric acid, and 81.51 parts (including 6.46 parts of N) of liquid ammonia to the tubular reactor and react quickly at 120°C (reaction time is about 1 second);
[0045] Step 3, with 284.8 parts of potassium chloride (containing K 2 O 170.88 parts), 99.6 parts of urea (containing 45.82 parts of N), 150 parts of ammonium dihydrogen phosphate (containing 16.5 parts of N, P 2 o 5 66 parts) were simultaneously added to the granulator, and the material in the tubular reactor and 14.39 parts of liquid ammonia (containing 11.73 parts of N) were sprayed into the granulator in an atomized state for mixing and granulation;
[0046] Step 4,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com