A kind of preparation method of 2-hydroxygulose receptor derivative, bleomycin disaccharide and its precursor
A technology of hydroxygulose and pyranoguloside, which is applied in the field of medicine and chemical industry, can solve the problems of unsuitable for industrialization, high cost, and scarce sources of natural L-gulose, and achieve easy control of conditions, high yield, highly operable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
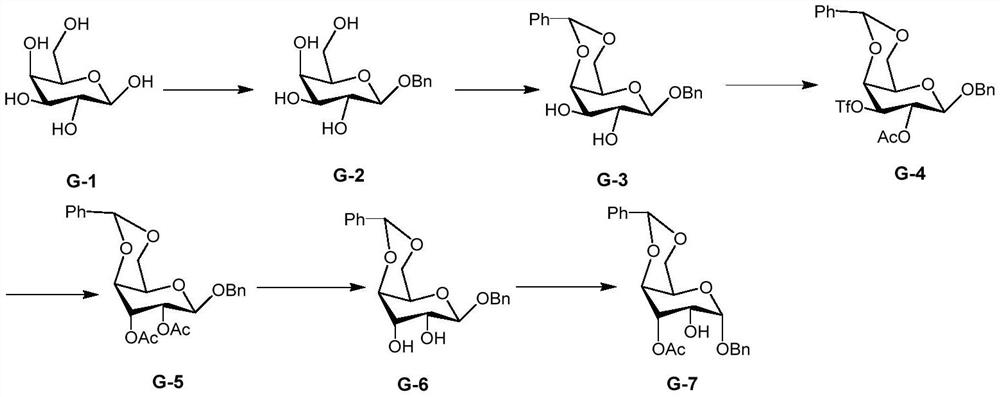
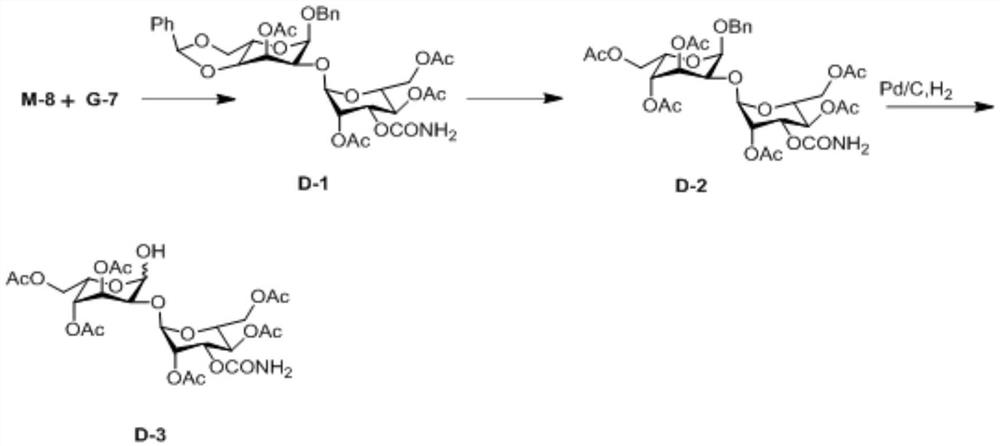
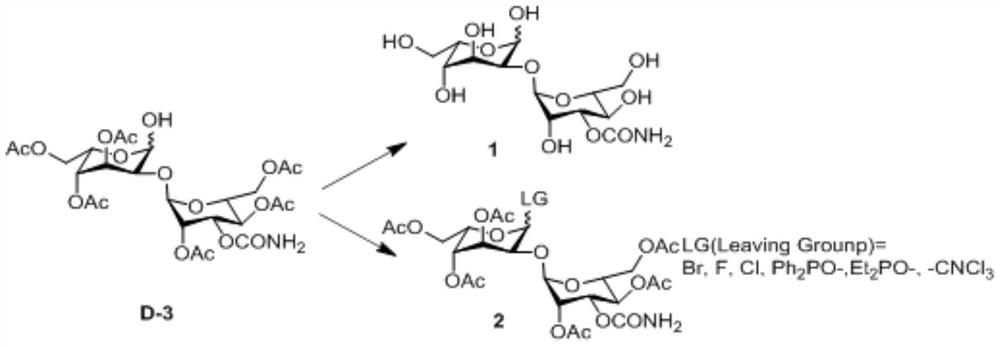
[0052] An embodiment of the preparation method of the 2-hydroxygulose receptor derivative of the present invention comprises the following steps:
[0053] (1) Preparation of benzyl-2,3-dihydroxy-4,6-O-benzylidene-β-D-galactopyranoside (G-3)
[0054] The G-3 structural formula is
[0055] ① Preparation of benzyl-β-D-galactopyranoside (G-2)
[0056] The G-2 structural formula is
[0057] Under the protection of nitrogen, dissolve 180g of β-D-galactopyranose (1mol) into 100mL of benzyl alcohol, add 1 g of p-toluenesulfonic acid to catalyze it at the same time, react overnight at 150°C, distill out the benzyl alcohol under reduced pressure, and use anhydrous methanol 235 g of benzyl-β-D-galactopyranoside were obtained by recrystallization, with a yield of 87.1%.
[0058] 1 H NMR (400MHz, CDCl 3 )δ7.50-7.20(m,5H),4.95(d,J=12Hz,1H),4.68(d,J=12Hz,1H),4.43(d,J=8.0Hz,1H),3.90-3.71( m,3H),3.68-3.40(m,3H).
[0059] ②Under the protection of nitrogen, add 27.5mL of benzene Form...
Embodiment 2
[0078] An embodiment of the preparation method of the 2-hydroxygulose receptor derivative of the present invention comprises the following steps:
[0079] (1) Preparation of benzyl-2,3-dihydroxy-4,6-O-benzylidene-β-D-galactopyranoside (G-3)
[0080] ① Preparation of benzyl-β-D-galactopyranoside (G-2)
[0081] Under the protection of nitrogen, dissolve 180g of β-D-galactopyranose (1mol) into 100mL of benzyl alcohol, add 1 g of p-toluenesulfonic acid to catalyze it at the same time, react overnight at 150°C, distill out the benzyl alcohol under reduced pressure, and use anhydrous methanol 235 g of benzyl-β-D-galactopyranoside were obtained by recrystallization, with a yield of 87.1%.
[0082] ②Under the protection of nitrogen, put 40.5g of benzyl-β-D-galactoside (150mmoL) and 2.85g of camphorsulfonic acid in dry dimethyl sulfoxide (DMSO) (250mL) solution, and drop 27.5mL of Benzaldehyde, the reaction mixture was stirred under reduced pressure for 4h, and the reaction temperatu...
Embodiment 3
[0092] An embodiment of the preparation method of the 2-hydroxygulose receptor derivative of the present invention comprises the following steps:
[0093] (1) Preparation of benzyl-2,3-dihydroxy-4,6-O-benzylidene-β-D-galactopyranoside (G-3)
[0094] ① Preparation of benzyl-β-D-galactopyranoside (G-2)
[0095] Under the protection of nitrogen, dissolve 180g of β-D-galactopyranose (1mol) into 100mL of benzyl alcohol, add 1 g of p-toluenesulfonic acid to catalyze it at the same time, react overnight at 150°C, distill out the benzyl alcohol under reduced pressure, and use anhydrous methanol 235 g of benzyl-β-D-galactopyranoside were obtained by recrystallization, with a yield of 87.1%.
[0096] ②Under nitrogen protection, add 27.5 mL of benzaldehyde dropwise to a solution of 40.5 g of benzyl-β-D-galactoside (150 mmoL) and 2.85 g of Dowex 50WX8-200 in dry diglyl dimethyl ether (250 mL) Dimethyl acetal (180mmoL), the reaction mixture was stirred under reduced pressure for 4h, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com