2,6-diiodo-BODIPY derivative and preparation method and application thereof
A derivative, diiodo technology, applied in the field of 2,6-diiodo BODIPY derivatives and its preparation, can solve the problems of incompatibility, complicated operation, low yield, etc., and achieve enhanced crossover efficiency and easy modification , The effect of improving photodynamic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (a) Synthesis of compound (I)
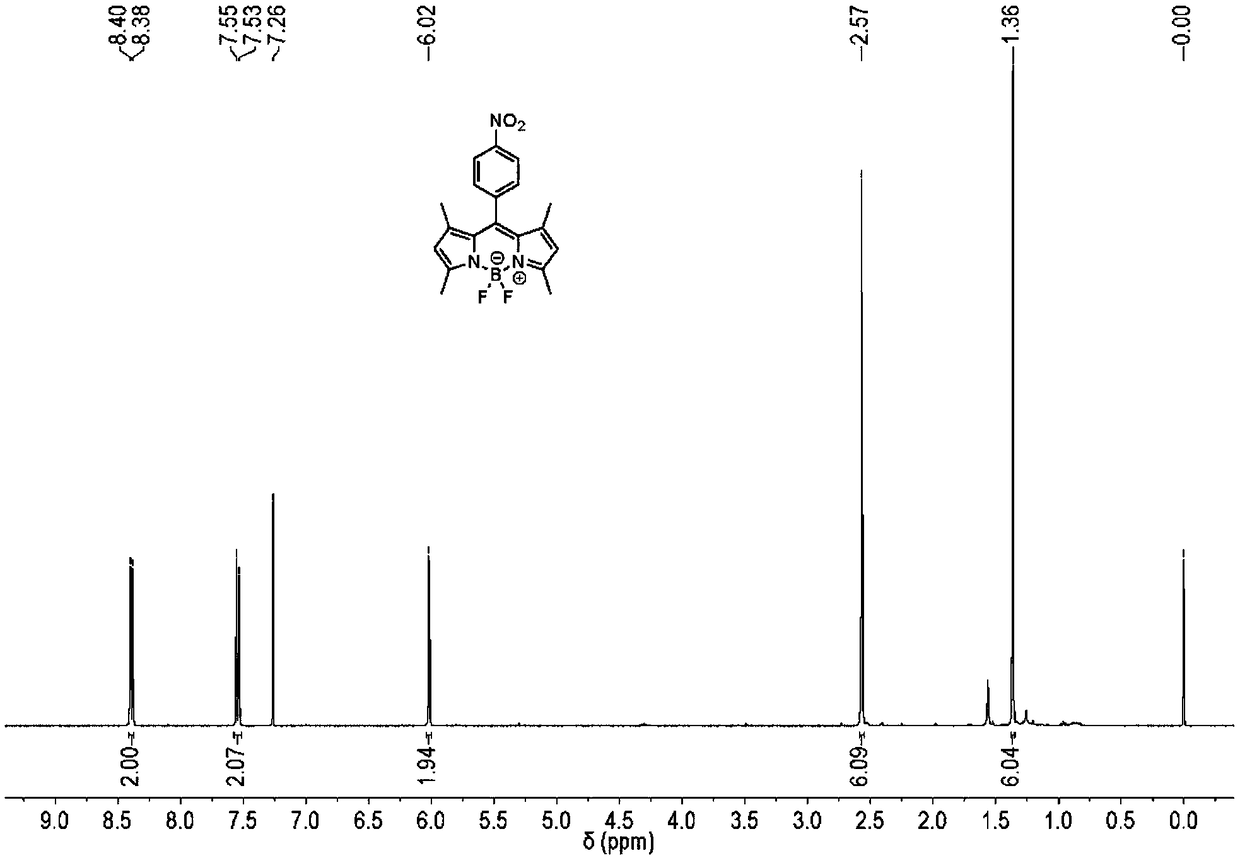
[0051] 4-Nitrobenzoyl chloride (1.0g) and 2,4-dimethylpyrrole (1.0g) were dissolved in dichloromethane (25mL), and heated at 45°C for 1h. Triethylamine (2.5 mL) was added and heating was continued at 45°C for 15 min. Boron trifluoride diethyl etherate (2.5 mL) was added and heated at 45° C. for 3 h. The mixture was evaporated to remove the solvent, and then separated by neutral alumina column chromatography with dichloromethane as the eluent. Purple-red solid, yield 0.8g, yield 40%
[0052] 1 H NMR (400MHz, CDCl 3 )δ8.39(d, J=8.7Hz, 2H), 7.54(d, J=8.7Hz, 2H), 6.02(s, 2H), 2.57(s, 6H), 1.36(s, 6H).
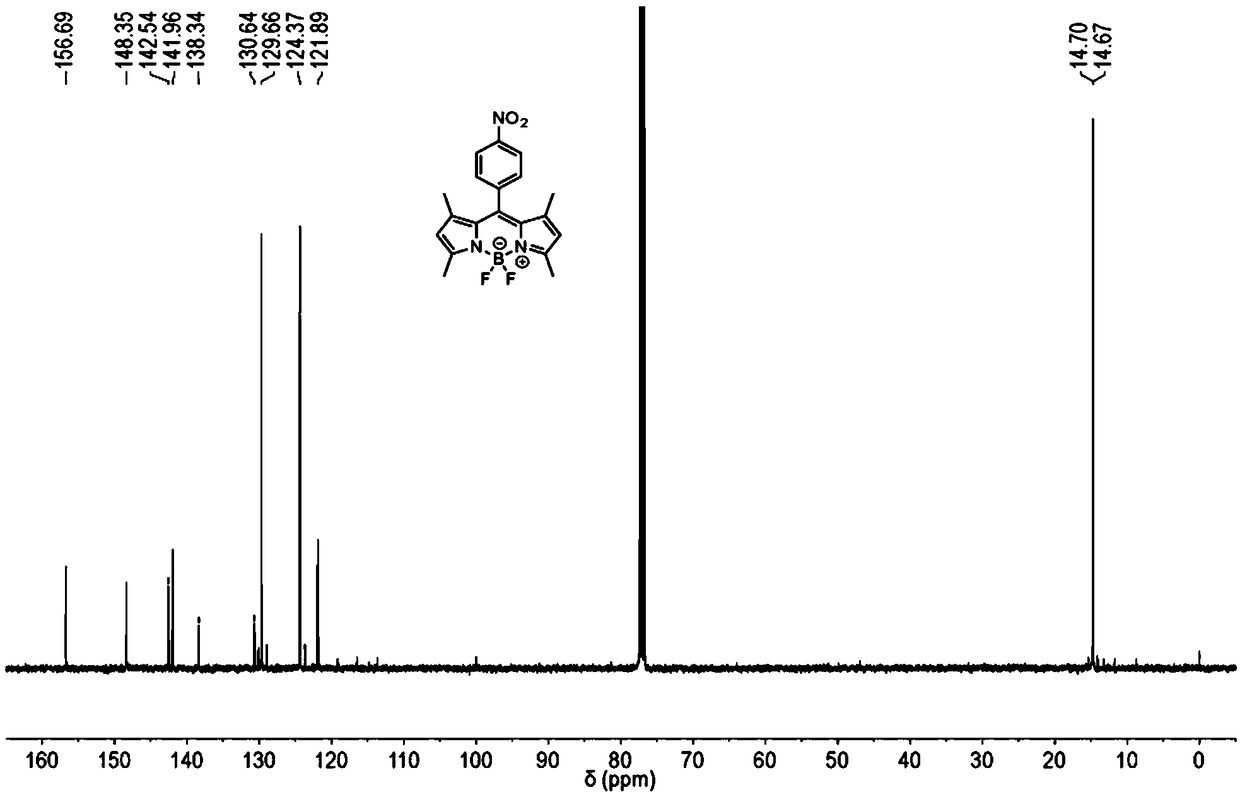
[0053] 13 C NMR (101MHz, CDCl 3 )δ156.69, 148.35, 142.54, 141.96, 138.34, 130.64, 129.66, 124.37, 121.86, 14.70, 14.67.
[0054] MALDI-TOF MS, Calcd. For[M], 369.146, Found, 369.703.
[0055] Anal. Calcd. For C 19 h 18 BF 2 N 3 o 2 (%): C, 61.82; H, 4.91; N, 11.38, Found: C, 61.65; H, 5.00; N, 11.28.
[0056] (b) Synthesis of co...
Embodiment 2
[0082] Surface Modification of COF LZU-1 by Compound III
[0083] Dissolve compound III (10.6 mg) in ethanol (5 mL), add COF LZU-1 (5 mg), and disperse uniformly by ultrasonic; then add aqueous acetic acid (50 μL, 3 mol / L), transfer to a polytetrafluoroethylene reactor, 75 ℃ insulation 4h. After cooling, centrifuge at 12000rpm for 30min, wash the precipitate with ethanol until the supernatant is colorless, then wash once with ether, and dry at 40°C to obtain orange-yellow powder COFLZU-1-BODIPY-2I.
[0084]The COF LZU-1 was prepared as follows: Trimylene (20 mg), p-phenylenediamine (20 mg), and trifluoroacetic acid (720 μL) were dissolved in ethanol (8 mL) to obtain a dark red solution. Transfer to a polytetrafluoroethylene reactor and keep warm at 120°C for 12h. After cooling, centrifuge at 12000rpm for 30min, wash the precipitate twice with ethanol+triethylamine (v / v=16:1) solution, then wash once with ether, and dry at 40°C to obtain a brownish yellow powder.
[0085] Th...
Embodiment 3
[0092] Heavy-atom-enhanced photodynamic performance
[0093] The COF LZU-1-BODIPY-2I was dispersed in phosphate saline buffer solution (PBS), and its photodynamic performance was evaluated by the specific singlet oxygen scavenger DPBF.
[0094] Take COF LZU-1-BODIPY-2I PBS dispersion (2mL, the concentration is 10μmol / L according to BODIPY), add DPBF / DMF solution (1mmol / L, 100μL), and use a green LED lamp (power density 40mW / cm 2 ) for different time, monitor the UV-Vis spectrum of DPBF ( Figure 14 ). The same method was used to evaluate the photodynamic performance of COF LZU-1-BODIPY with the same concentration ( Figure 15 ). And take the maximum absorption peak absorbance A of DPBF and the initial absorbance A 0 The ratio of A / A 0 is the ordinate, and the illumination time is plotted as the abscissa ( Figure 16 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com