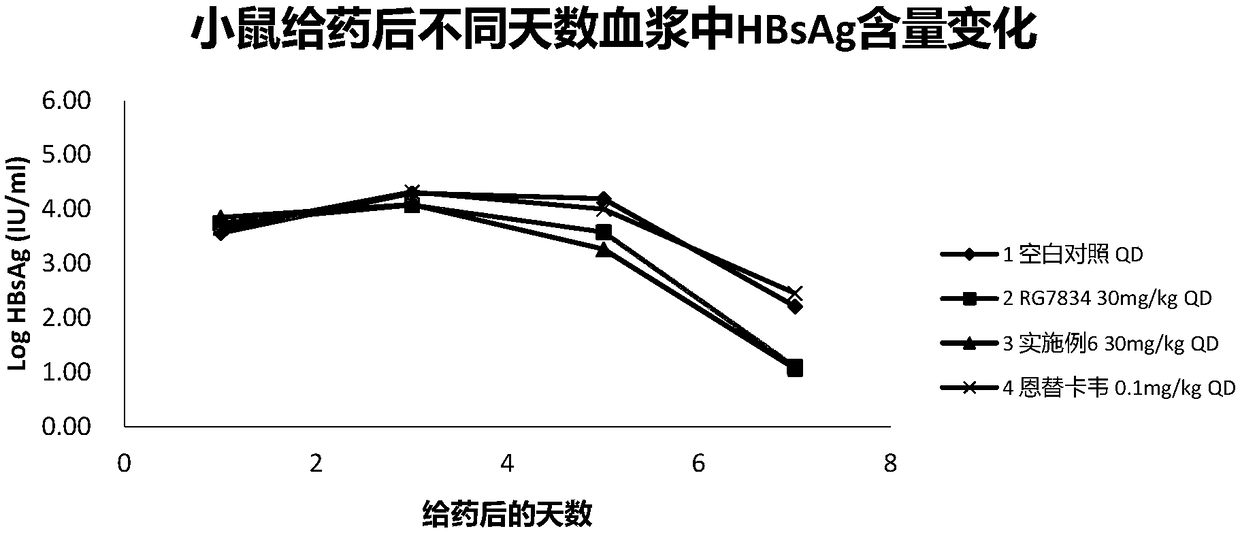
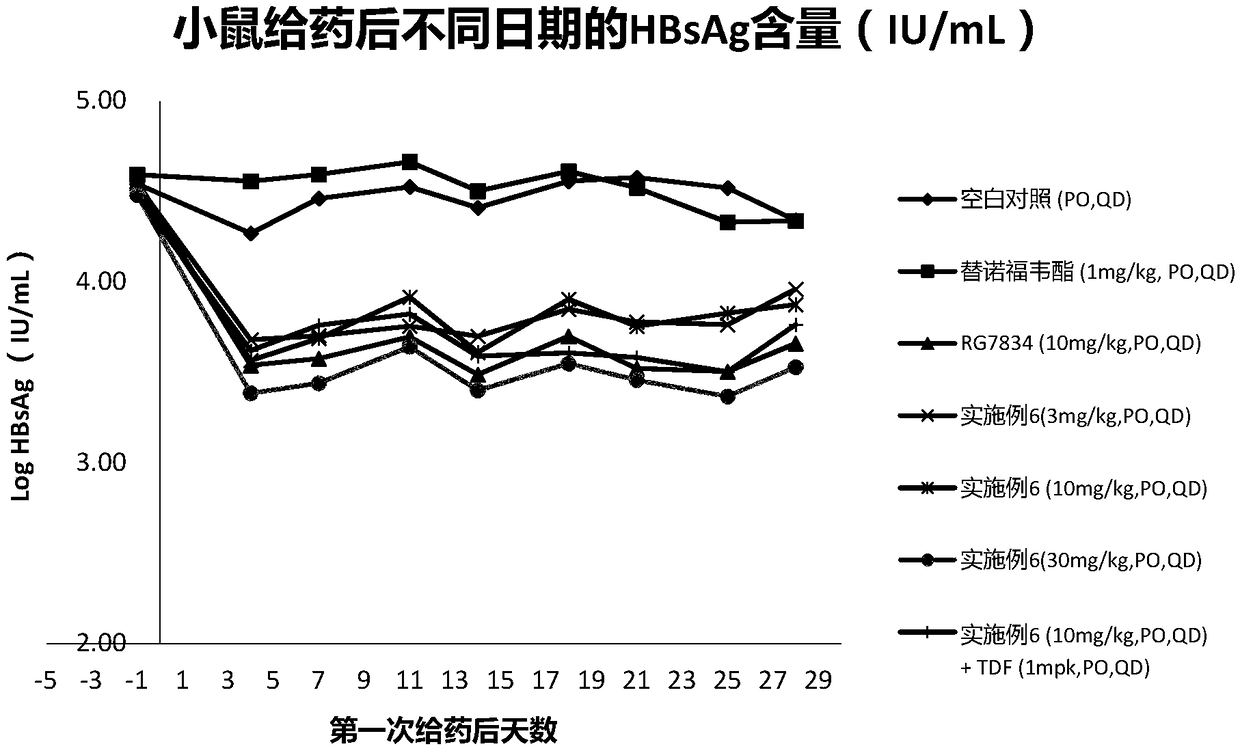
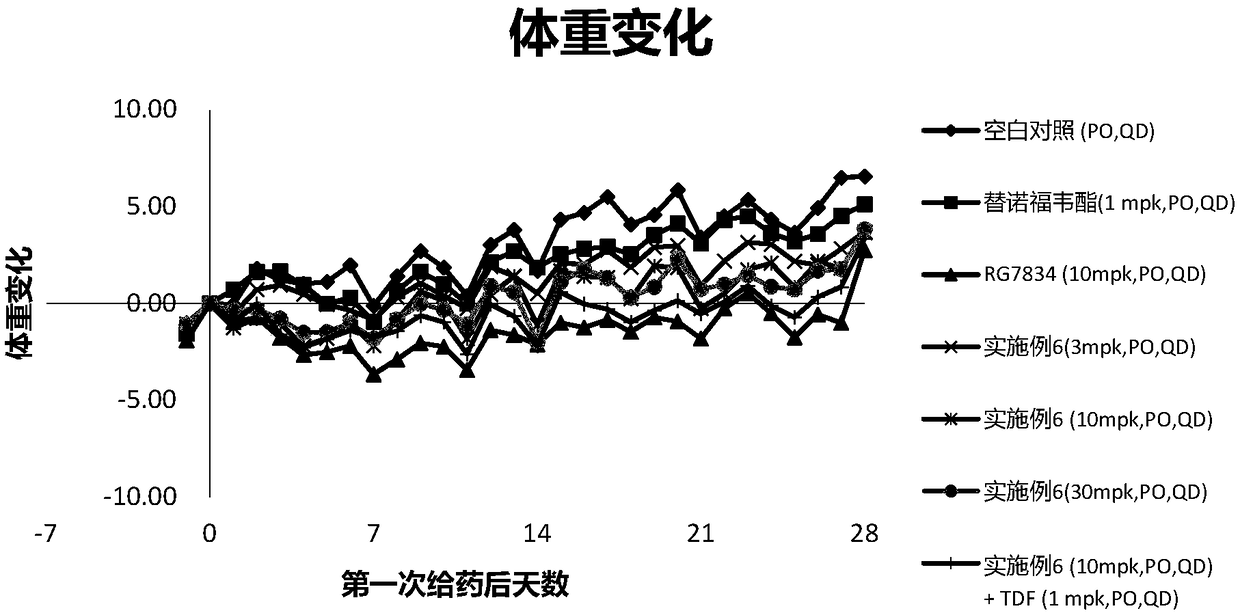
Hepatitis B virus surface antigen inhibitor
A technology of atoms and carbon atoms, applied in the field of hepatitis B virus surface antigen inhibitors, can solve the problem of poor water solubility of 7-membered carbon rings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181]
[0182] Step A: 1-1 (200.00 g, 1.19 mol) was dissolved in N,N-dimethylformamide (1000.00 ml), potassium carbonate (164.39 g, 1.19 mol) was added, and then added dropwise at 90°C for one hour 1-Bromo-3-methoxy-propane (182.01 g, 1.19 mol). The mixture was stirred at 90°C for 15 hours. Add 3 liters of ethyl acetate (1 liter × 3) to the reaction solution, combine the organic phases, wash with 15.00 liters of water (3.00 liters × 5), and 3.00 liters of saturated brine (1.00 liters × 3), and dry over anhydrous sodium sulfate , and concentrated under reduced pressure to obtain a crude product. Then purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=1 / 0) to obtain compound 1-2.
[0183] Step B: 1-2 (80.00 g, 332.98 mmol) was dissolved in acetonitrile (800.00 mL), then chlorosuccinimide (44.46 g, 332.98 mmol) was added and the mixture was stirred at 90°C for three hours. First spin off half the volume of acetonitrile, then add 200.00 ml o...
Embodiment 2
[0199]
[0200] ee value (enantiomeric excess): 100%.
[0201] SFC (Supercritical Fluid Chromatography) Method: Column: Chiral pak AD-3 100 mm x 4.6 mm I.D., 3 microns. Mobile phase: methanol (0.05% diethylamine) from 5% to 40% in carbon dioxide. Flow rate: 3 ml per minute. Wavelength: 220 nm.
[0202] 1 H NMR (400MHz, deuterated chloroform) δ15.77(br s, 1H), 8.49(s, 1H), 7.52(s, 1H), 6.87(s, 1H), 6.68(s, 1H), 4.49-4.72 (m, 2H), 4.12-4.28(m, 2H), 3.92(br d, J=6.40Hz, 1H), 3.63(t, J=5.90Hz, 2H), 3.39(s, 3H), 2.06-2.24 (m, 3H), 1.10 (d, J=6.53Hz, 3H), 0.89 (d, J=6.53Hz, 3H).
Embodiment 3
[0204]
[0205] ee value (enantiomeric excess): 100%.
[0206] SFC (Supercritical Fluid Chromatography) Method: Column: Chiral pak AD-3 100 mm x 4.6 mm I.D., 3 microns. Mobile phase: methanol (0.05% diethylamine) from 5% to 40% in carbon dioxide. Flow rate: 3 ml per minute. Wavelength: 220 nm.
[0207] 1 H NMR (400MHz, deuterated chloroform) δ15.81(s, 1H), 8.88-9.18(m, 1H), 7.47(s, 1H), 6.78(s, 1H), 6.76(s, 1H), 4.48- 4.59(m, 2H), 4.19(dt, J=2.07, 6.12Hz, 2H), 3.59-3.67(m, 2H), 3.45-3.52(m, 1H), 3.39(s, 3H), 2.15(quin, J=6.05Hz, 2H), 1.14-1.31(m, 2H), 0.29-0.55(m, 2H), 0.09(s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com