Compound for detecting cell autophagy enhancement as well as preparation method and application thereof
A compound and cell technology, applied in the fields of compounds containing group 3/13 elements of the periodic table, chemical instruments and methods, organic chemistry, etc., can solve the problem of inability to indicate enhanced autophagy, poor membrane permeability of metal complexes, etc. problems, to achieve the effect of high-throughput screening, excellent cell permeability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
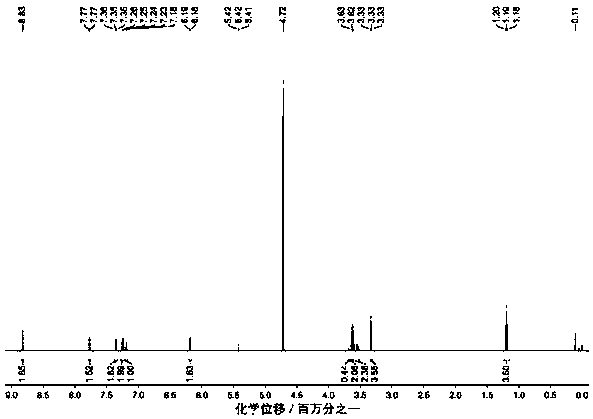
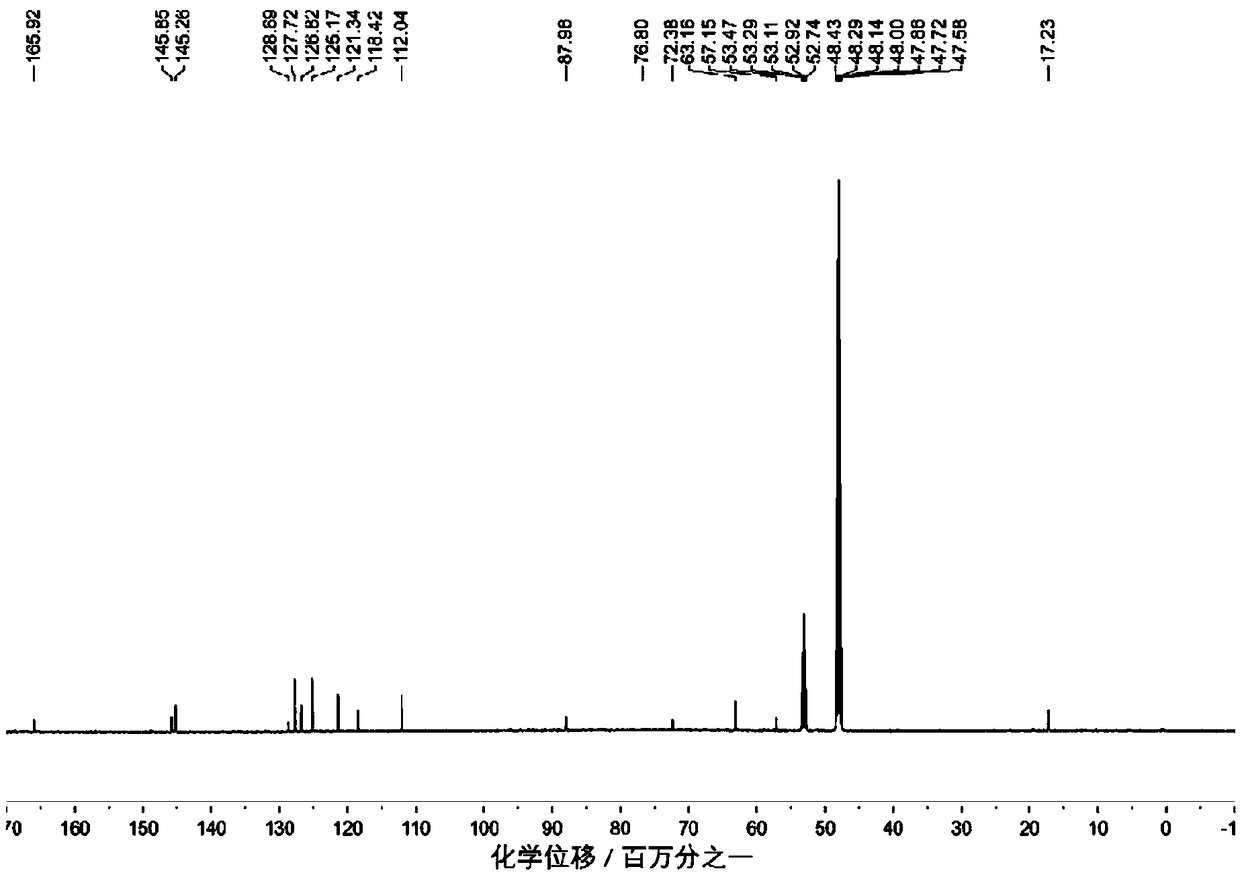
[0044] Embodiment 1: the synthesis of compound SN1
[0045] Add (R1=R2=H) benzaldehyde (0.106g, 1mmol) and 2-cyano-2-(ethoxycarbonylvinyl)-1-H-pyrrole (0.380g, 2mmol) into the reaction vessel, dissolve in 50ml of anhydrous dichloromethane. Extract 0.5ml of trifluoroacetic acid (TFA) catalyst into the system with a syringe, put into a magnetic stirrer to stir under anhydrous and dark conditions, and monitor with a thin-layer chromatographic plate after reflux for 7 days until the described 2-cyano-2-(B Oxycarbonylvinyl)-1-H-pyrrole was completely consumed. Then 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ: 0.227g, 1mmol) was added to the reaction solution, stirred at room temperature for 30 minutes, and then triethylamine (TEA) was added After stirring 3ml evenly, add boron trifluoride-ether solution (BF 3 ·Et 2 (2) 6ml (mass percentage 48%), continue stirring and reflux reaction for 1 hour, obtain the blue solution with red fluorescence, point plate monitoring until the ...
Embodiment 2
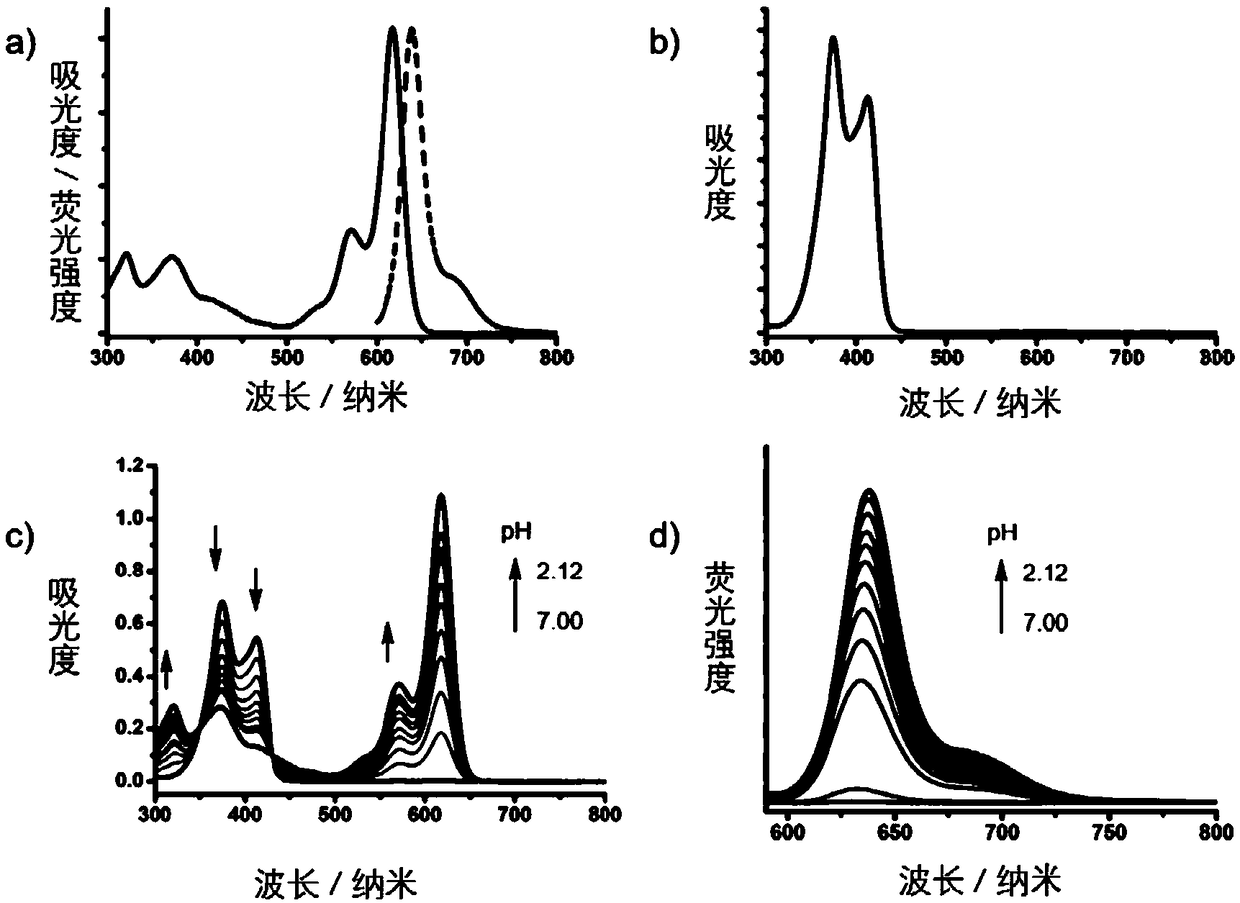
[0046] Embodiment 2: pH titration experiment of SN1
[0047] Compound SN1 is a colorless to pale yellow solution in methanol, and its UV absorption spectrum is shown in image 3, there are two absorption peaks at 374 nm and 413 nm. TFA was added to the methanol solution of iso-BODIPY for pH titration, and an obvious color change from light yellow to red was observed during the titration from pH 7.0 to 2.12, accompanied by strong red fluorescence. When the pH value is below 6.0, the generation of fluorescence begins to be observed, and when the pH value changes from 7.0 to 4.1, the fluorescence emission intensity (λem=635nm) increases by more than 17 times. image 3 The inset in shows the change in absorption at 618 nm during the pH titration. A pKa value of 4.71 was obtained by using the Henderson-Hasselbach equation. The changes of ultraviolet absorption spectrum and fluorescence spectrum indicated that iso-BODIPY is suitable for pH fluorescent probe and may have the poten...
Embodiment 3
[0048] Example 3: Lysosome fluorescent labeling and real-time monitoring application of SN1
[0049] The KB cell line was provided by the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. KB cells were cultured in serum-free freezing medium (RPMI) 1640 supplemented with 10% fetal bovine serum (FBS). Place it in a carbon dioxide incubator at 37°C for 4 hours. Cells can be directly scanned and imaged without washing. The synthesized iso-BODIPY and labeled lysosomal fluorescent probe were further studied by 3D live cell imaging technology. Hela cells were loaded with 1 (15 μM) for 30 minutes and observed under a confocal microscope, then rapamycin (20 μM) was added under the microscope. For each field, a series of 10-20 images of successive focal planes, separated by 0.8 μm, were recorded and plotted against the vertical projection of each focal plane. Record fluorescence images at selected time points and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com