Isopropyl alcohol bridged sulfonazole type compound as well as preparation method and application thereof
An isopropanol bridged, sulfonylazole technology, applied in the direction of organic chemistry, resistance to vector-borne diseases, antibacterial drugs, etc., to achieve the effect of short synthesis route, simple preparation of raw materials, and resolution of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
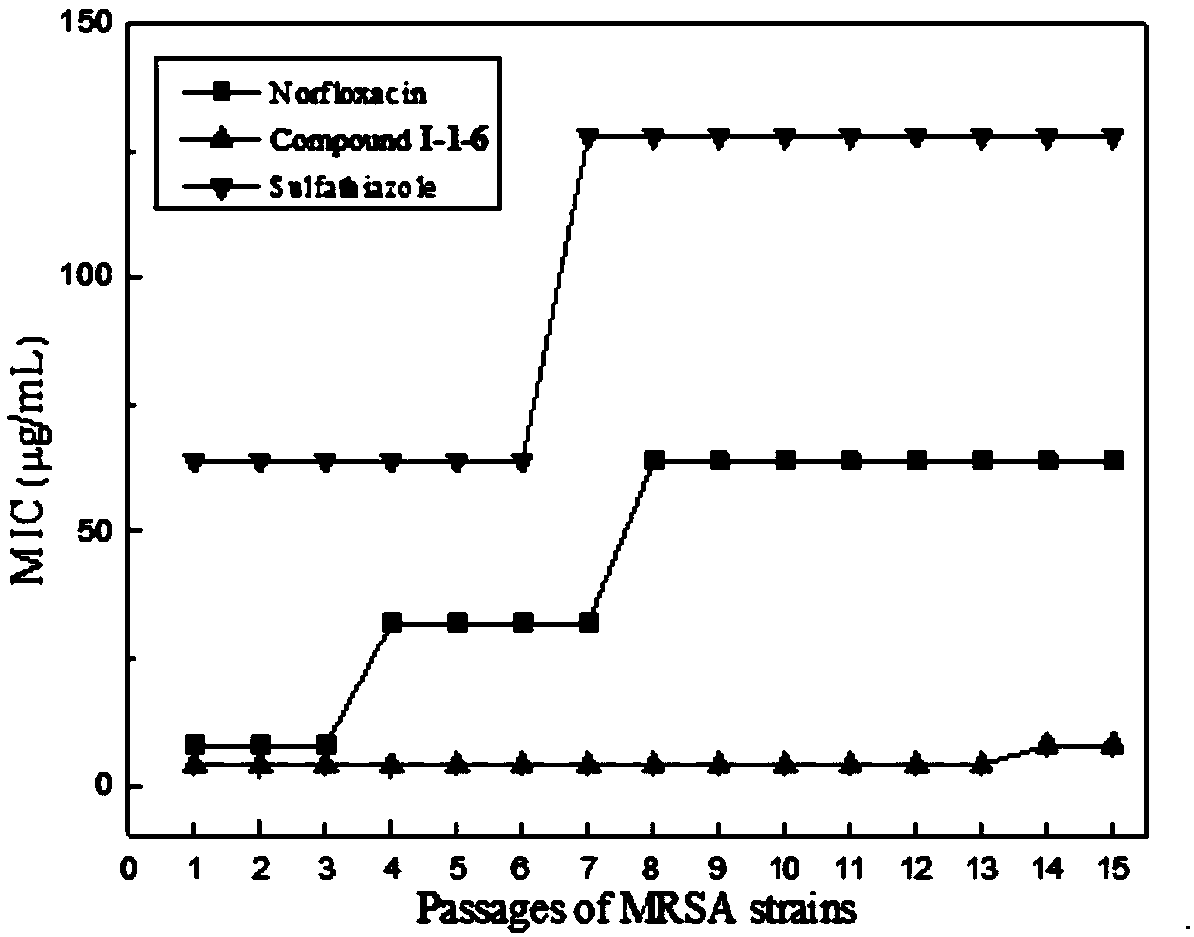
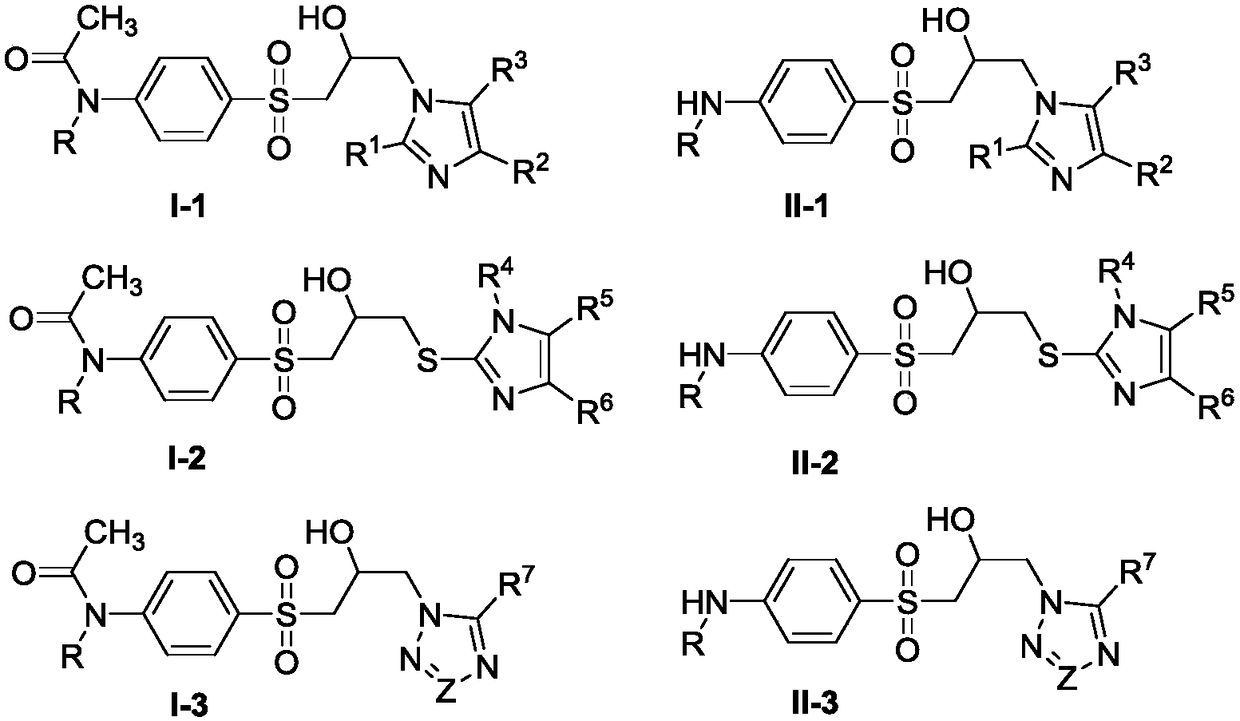
Image
Examples
Embodiment 1
[0042] Preparation of Intermediate III
[0043]
[0044] Add sodium p-acetamidobenzenesulfonate (4.73g, 21.40mmol) and tetrabutylammonium iodide (0.01g, 0.03mmol) into a 150mL round-bottomed flask, with epichlorohydrin (30mL) as solvent, at 80°C The reaction was stirred, followed by TLC until the end of the reaction. The solvent was spin-dried under reduced pressure, and the residue was made of ethyl acetate as an extractant. The organic phase was collected and dried, and concentrated to obtain Intermediate III (2.03g), with a yield of 37.1%; white solid; melting point: 170-171 ℃; 1 H NMR (600MHz, DMSO-d 6 )δ10.38(s,1H,NH),7.83–7.79(m,4H,Ph-2,3,5,6-H),4.14(s,1H,SO 2 CH 2 ),4.08(s,1H,SO 2 CH 2 ),3.65–3.62(m,2H,CHOCH 2 ),3.58–3.55(m,1H,CHOCH 2 ),2.10(s,3H,CH 3) ppm; 13 C NMR (151MHz, DMSO-d 6 )δ 169.6, 144.4, 134.0, 129.7, 129.5, 119.0, 113.4, 66.3, 59.9, 49.0, 24.6ppm.
Embodiment 2
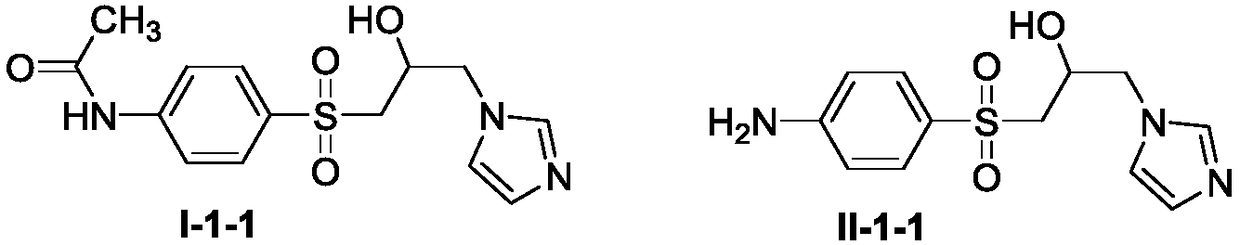
[0046] Preparation of compound I-1-1
[0047]
[0048] Add imidazole (0.14g, 2.04mmol) to a 150mL round bottom flask, use potassium carbonate as base (0.32g, 2.35mmol), acetonitrile as solvent, stir at 70°C for 30 minutes, cool to room temperature, add intermediate III (0.40 g, 1.57mmol) continued to heat up to 70°C for reaction, followed by TLC until the end of the reaction. After concentration, extraction, column chromatography separation, drying and other post-treatments, compound I-1-1 (0.42 g) was obtained, with a yield of 82.7%; white solid; melting point: 216-218°C; 1 HNMR (600MHz, DMSO-d 6 )δ10.35(s,1H,NH),7.72(d,J=8.8Hz,2H,Ph-2,6-H),7.65(d,J=8.8Hz,2H,Ph-3,5-H ),7.53(s,1H,Im-2-H),7.08(s,1H,Im-5-H),6.70(s,1H,Im-4-H),5.19(s,1H,OH), 4.54–4.50(m,1H,CHOH),3.99–3.95(m,1H,Im-CH 2 ), 3.76 (dd, J=15.1, 3.5Hz, 1H, Im-CH 2 ),3.59–3.52(m,2H,SO 2 CH 2 ),2.09(s,3H,COCH 3 ) ppm; 13 C NMR (150MHz, DMSO-d 6 )δ169.6, 144.4, 137.3, 133.0, 129.1, 128.6, 119.0, 118.2, 64.2, 56...
Embodiment 3
[0050] Preparation of Compound II-1-1
[0051]
[0052] Add compound I-1-1 (0.10g, 0.31mmol) and 5mL ethanol in a 50mL round-bottomed flask as a solvent, add dropwise a 40% hydrochloric acid solution (5mL), reflux at 90°C, follow up to the reaction by TLC Finish. After cooling to room temperature, the reaction mixture was diluted with distilled water (15 mL) and washed with saturated NaHCO 3 Solution neutralization. After filtration and precipitation, the desired compound II-1-1 (0.17g) was obtained with a yield of 95.3%; white solid; melting point was 243-245°C; 1 H NMR (600MHz, DMSO-d 6 )δ7.53(s,1H,Im-2-H),7.35(d,J=8.6Hz,2H,Ph-3,5-H),7.11(s,1H,Im-5-H),6.75 (s,1H,Im-4-H),6.57(d,J=8.6Hz,2H,Ph-2,6-H),6.15(s,2H,NH 2 ), 5.38 (s, 1H, OH), 4.46 (d, J=4.9Hz, 1H, CHOH), 3.76–3.72 (m, 1H, Im-CH 2 ), 3.64 (dd, J=14.9, 3.8Hz, 1H, Im-CH 2 ),3.60–3.47(m,2H,SO 2 CH 2 ) ppm; 13 C NMR (150MHz, DMSO-d 6 )δ154.2, 137.3, 129.8, 128.6, 118.3, 113.3, 64.2, 57.3, 54.9ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com