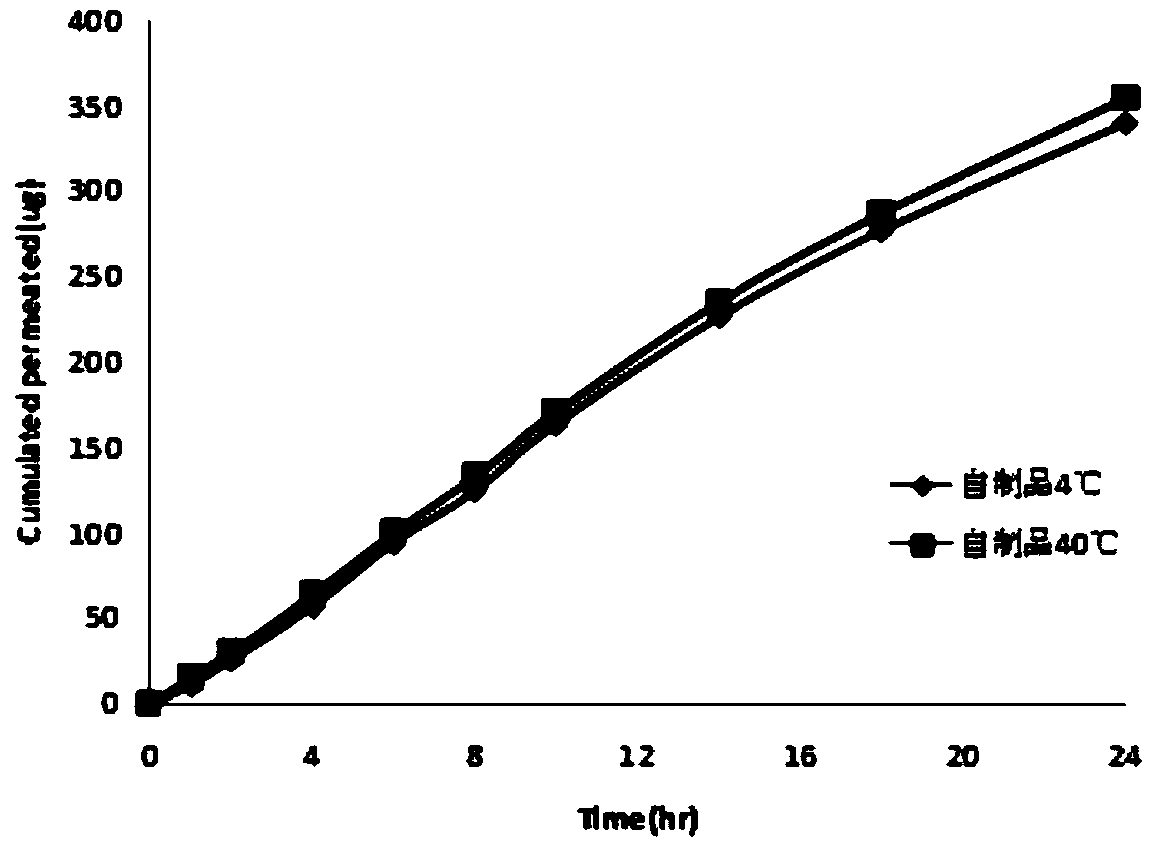
Tulobuterol transdermal patch and preparation method thereof
A transdermal patch, tulobuterol technology, applied in the field of tulobuterol transdermal patch and its preparation, can solve the problems of slow initial transdermal rate, easy crystallization, poor stability of microcrystalline drugs, etc., to achieve The effect of reducing the difficulty of industrial production, excellent stability, and reducing the difficulty of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The consumption of each raw material is as follows in the present embodiment:
[0044] Drug solution:
[0045]
[0046] Drug storage:
[0047] Drug solution 200mL
[0048] Carbomer 934 25g
[0049] Silicone pressure sensitive adhesive 10g
[0050] Backing film: multi-layer composite aluminum foil.
[0051] Preparation Process:
[0052] Preparation of drug solution: Take polysorbate, isopropanol, oleic acid and tulobuterol according to the prescription amount, dissolve them all under stirring at room temperature, then continue stirring, and add deionized water to the total amount to obtain the drug solution solution.
[0053] Preparation of the drug storage: add the prescribed amount of carbomer to the drug solution prepared above to fully swell, stir at room temperature for 4 hours, and then let it stand overnight; add the prescribed amount of pressure-sensitive adhesive and continue stirring for 2 hours, then coat Cloth the reservoir layer and dry the reservo...
Embodiment 2
[0056] The consumption of each raw material is as follows in the present embodiment:
[0057] Drug solution:
[0058]
[0059] Drug storage:
[0060] Drug solution 200mL
[0061] Carbomer 1342 18g
[0062] Silicone pressure sensitive adhesive 10g
[0063] Backing film: multi-layer composite aluminum foil.
[0064] Preparation of the drug solution: take polysorbate, isopropanol, stearic acid and tulobuterol according to the prescription amount, dissolve them all under stirring at room temperature, then continue to stir, and add deionized water to the total amount, obtain a drug solution;
[0065] Preparation of the drug storage: add the prescribed amount of carbomer to the drug solution prepared above to fully swell, stir at room temperature for 5 hours, and then let it stand overnight; add the prescribed amount of pressure-sensitive adhesive and continue stirring for 3 hours, then coat Cloth the reservoir layer and dry the reservoir layer.
[0066] Tulobuterol patch ...
Embodiment 3
[0068] The consumption of each raw material is as follows in the present embodiment:
[0069]
[0070] Drug storage:
[0071] Drug solution 200mL
[0072] Carbomer 934 20g
[0073] Silicone pressure sensitive adhesive 5g
[0074] Backing film: multi-layer composite aluminum foil.
[0075] Preparation Process:
[0076] Preparation of the drug solution: take polysorbate, isopropanol, oleic acid and tulobuterol according to the prescription amount, dissolve them all under stirring at room temperature, then continue to stir, and add deionized water to the total amount to obtain drug solution;
[0077] Preparation of the drug storage: add the prescribed amount of carbomer to the drug solution prepared above to fully swell, stir at room temperature for 4.5 hours, and then let it stand overnight; add the prescribed amount of pressure-sensitive adhesive and continue stirring for 2.5 hours, then coat Cloth the reservoir layer and dry the reservoir layer.
[0078] Tulobuterol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com