Phase-change mesoporous silicon bionic preparation for targeting controlled release medicine as well as preparation method and application thereof
A mesoporous silicon and preparation technology, which is applied in the field of phase-change mesoporous silicon biomimetic preparations and its preparation, can solve the problems of inability to eliminate cell carriers, incapable of active controlled drug release, and narrow application range, and achieve flexible combination and powerful loading. Medicinal ability, the effect of easy surface modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation method of drug-loaded phase-change mesoporous silicon biomimetic preparation
[0042] 1. Synthesis of drug-loaded phase-change mesoporous silicon biomimetic preparations
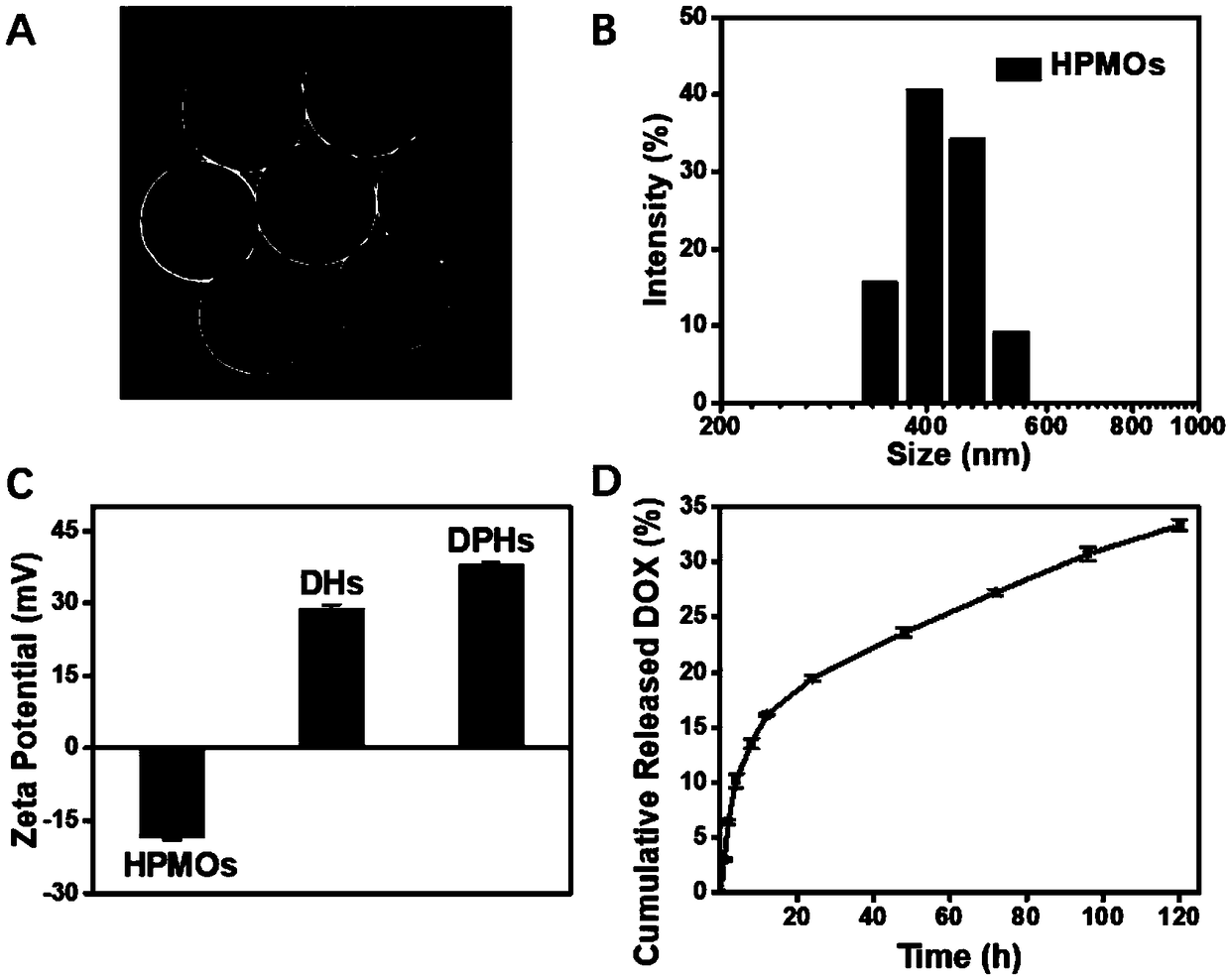
[0043](1) Take by weighing 1.2027g cetyltrimethylammonium bromide (Cetrimonium Bromide, CTAB), add 20mL deionized water and 10mL absolute ethanol, be made into the CTAB solution of 0.11mol / L concentration, in the situation of stirring The silicon dioxide solution was added gradually under the condition of adding 3 mL of ammonia water. After reacting for 5 minutes, 3 mL of 1,4-bis(triethoxysilyl)benzene (1,4-bis(triethoxysilyl)benzene, BTEB) was added and stirred for another 6 h. Centrifuge at 15000rpm / min for 15 minutes to collect the product, and disperse the precipitate in 0.6mol / L Na 2 CO 3 solution, and magnetically stirred in a water bath at 80°C for 30 minutes for etching. The product was collected by centrifugation at 15,000 rpm / min for 15 minutes, washed three times w...
Embodiment 2
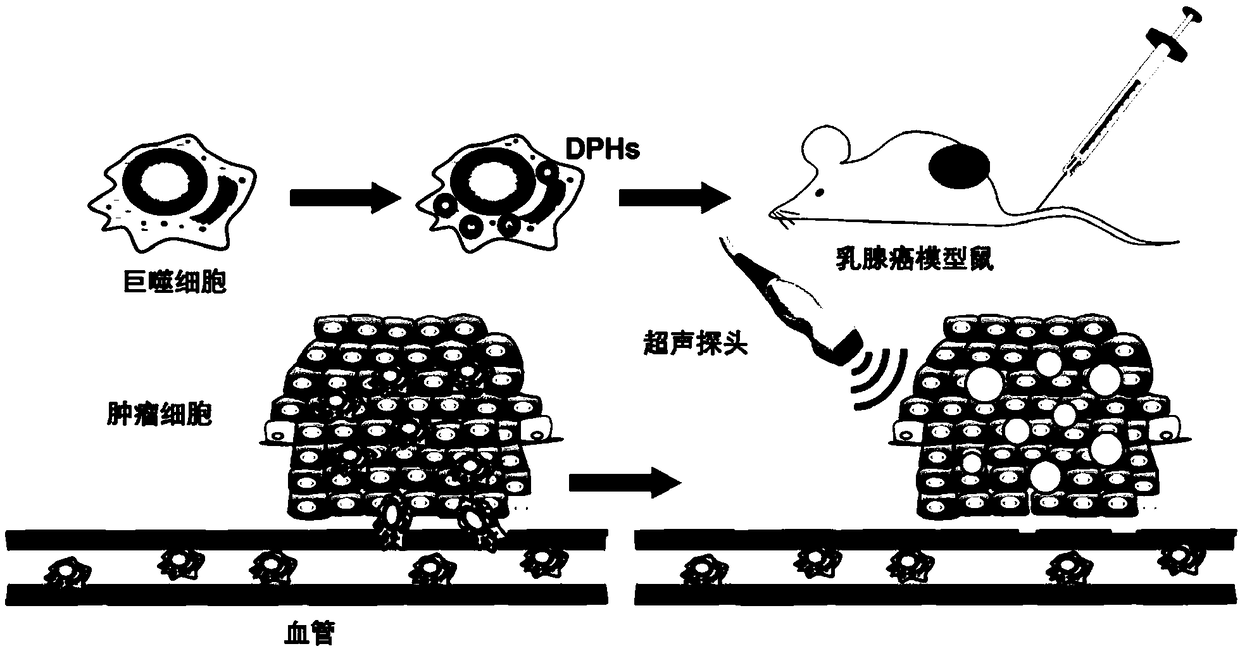
[0055] Example 2 Drug-loaded phase-change mesoporous silicon preparations (Cell-DPHs) are used for the treatment of breast cancer mice
[0056] 1. Construction of 4T1 breast cancer mouse model
[0057] (1) The inventor selected Balb / c nude mice as model mice, injected 4T1 cells subcutaneously into the right axillary fat pad of the mice at a ratio of 1 million 4T1 cells per mouse, and measured the tumor every day after 3 days volume.
[0058] (2) When the tumor volume reaches 150mm 3 At that time, the breast cancer model mice were divided into groups and started Cell-DPHs treatment. The inventors randomly divided them into 5 groups according to tumor volume and shape, with 6 rats in each group. The specific groups are as follows: control group (Control group), ultrasound group (US group), ultrasound phase change group (US+Cell-PHs group), drug-loaded phase-change group (Cell-DPHs group) and ultrasound-loaded phase-change group (US+Cell-DPHs group).
[0059] 2. Therapeutic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com