A kind of α-manganese dioxide and its preparation method and a kind of electrocatalyst
A manganese dioxide and manganese sulfate technology, applied in manganese oxide/manganese hydroxide, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc. control and affect the stability of the electrocatalytic performance of α-MnO, and achieve the effects of excellent oxygen reduction and oxygen evolution catalytic performance, mild reaction conditions, and good electrocatalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of α-manganese dioxide, comprises the following steps:
[0027] Mix manganese sulfate, potassium permanganate, water and hydrochloric acid solution, and conduct a hydrothermal reaction at 100-180°C for 0.5-72 hours to obtain α-manganese dioxide; the Mn in the α-manganese dioxide 3+ with Mn 4+ The mass ratio is 0.5 to 1.0.
[0028] In the present invention, the molar ratio of the manganese sulfate to potassium permanganate is preferably 10-15:6-10, more preferably 12-13:7-8; the manganese sulfate is preferably manganese sulfate monohydrate. In the present invention, the mass fraction of the hydrochloric acid solution is preferably 35 to 40%; the volume of the hydrochloric acid solution and the molar ratio of potassium permanganate are preferably 5 to 6 mL: 6 to 10 mmol; the hydrochloric acid solution to water The volume ratio is preferably 3-5:70-90.
[0029] In the present invention, the mixing is preferably to firs...
Embodiment 1
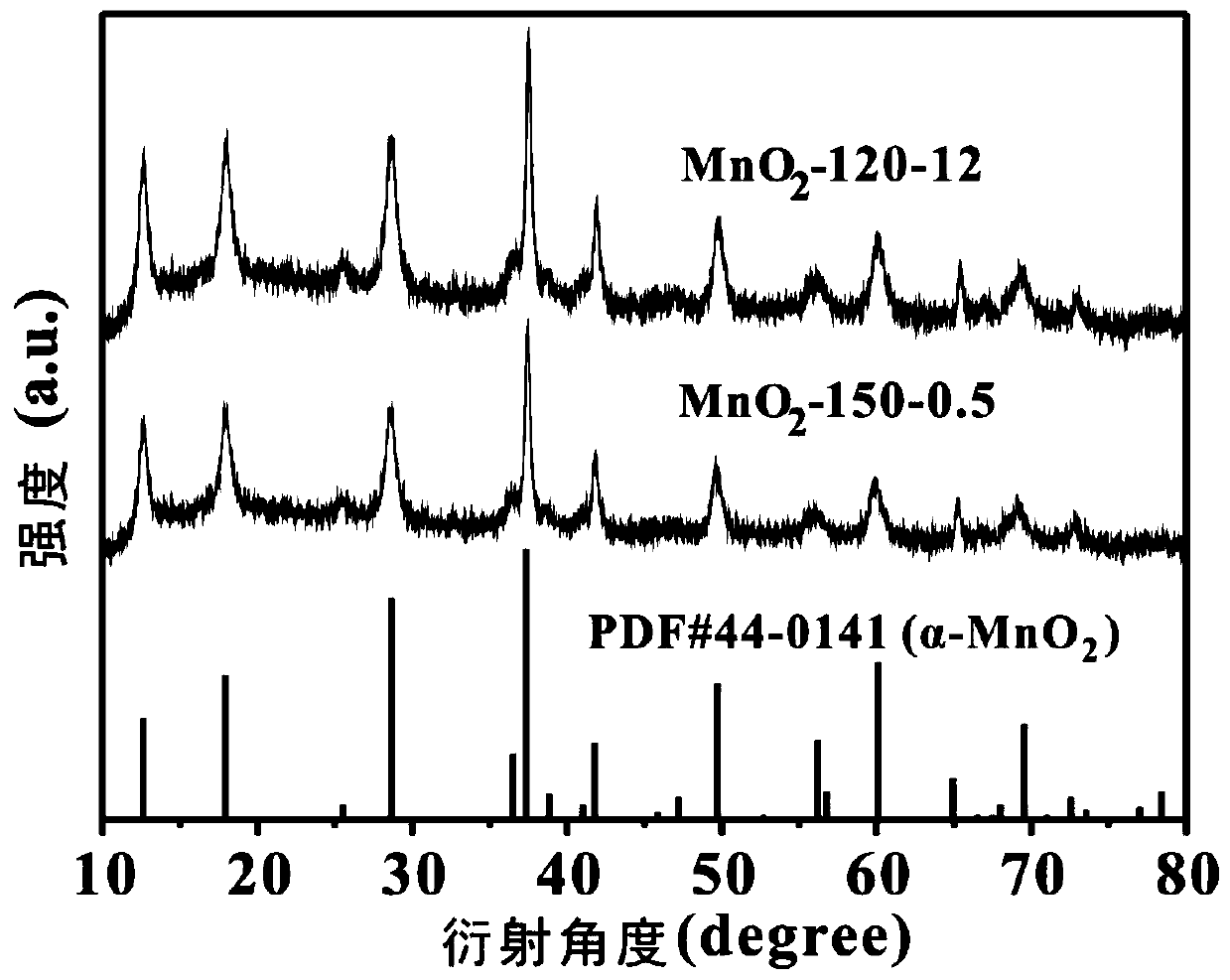
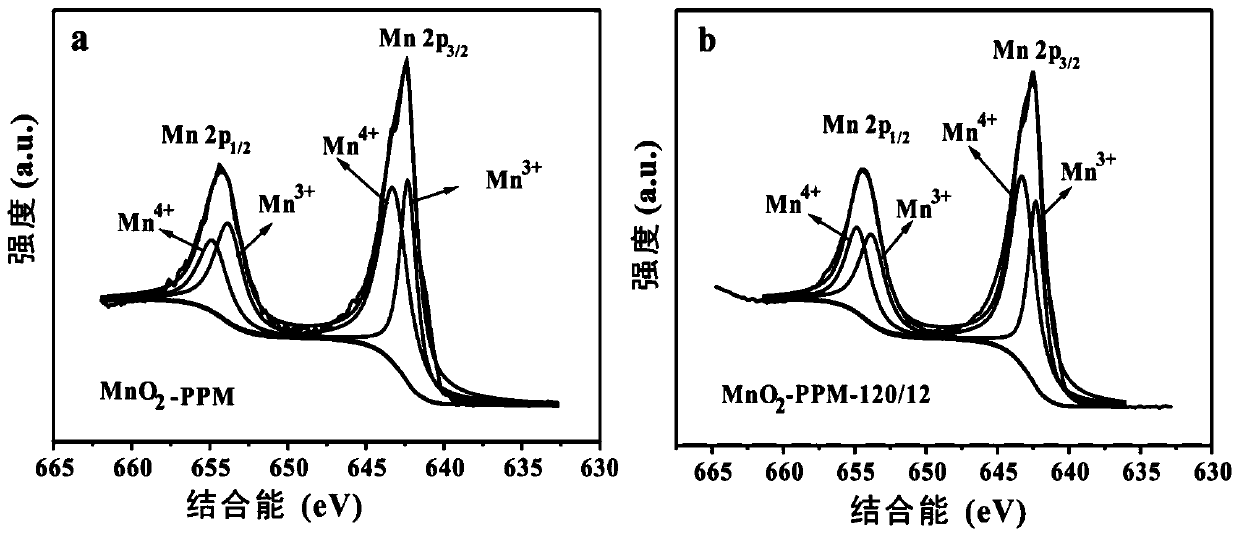
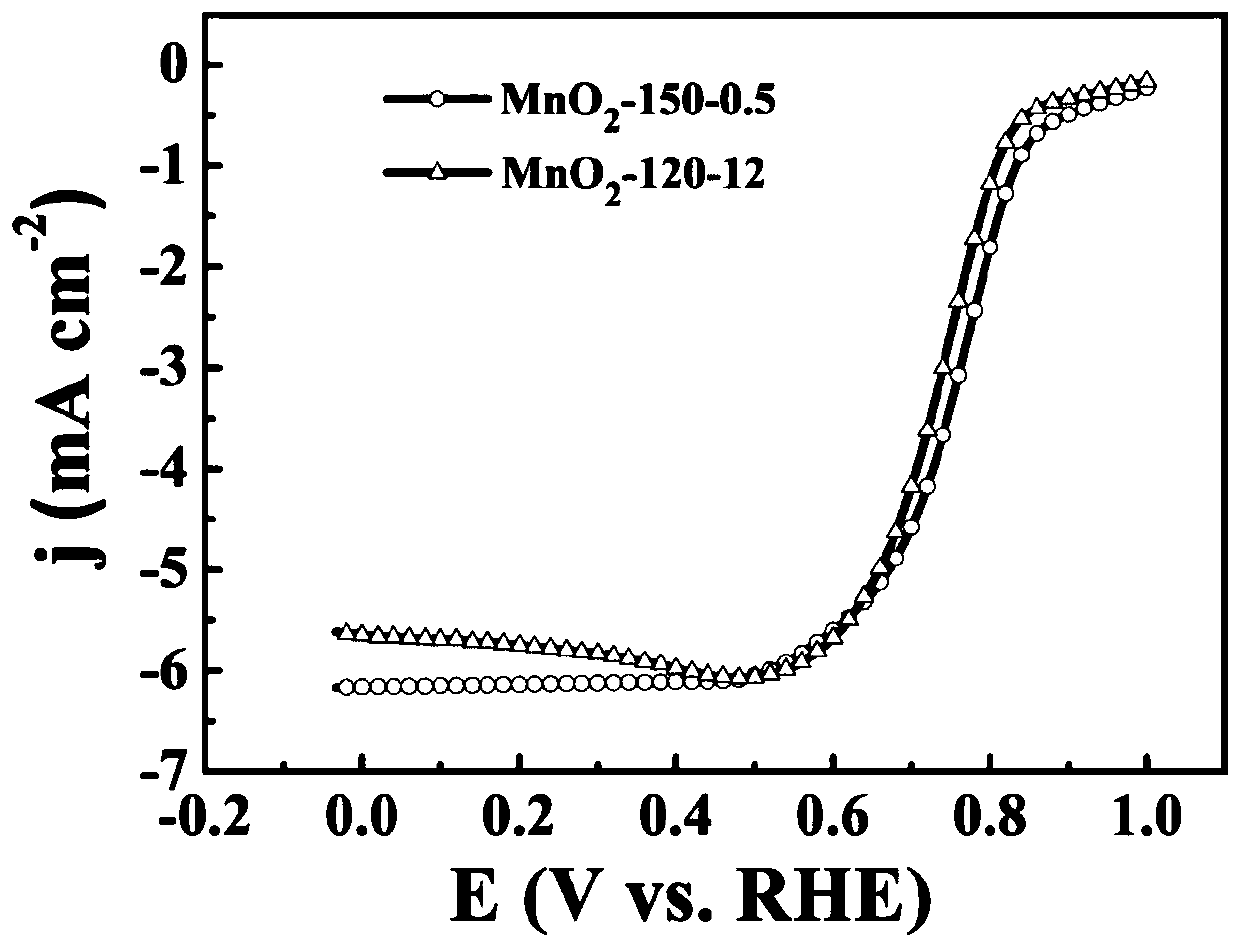
[0044] Dissolve 2.04g of manganese sulfate monohydrate and 1.27g of potassium permanganate in 80mL of deionized water, stir magnetically for 15min, then add 4mL of hydrochloric acid solution with a mass concentration of 37%, and stir for 5min to obtain a mixed solution; transfer the mixed solution Put it into a 100mL hydrothermal reaction kettle with a stainless steel shell and a polytetrafluoroethylene liner, and place the hydrothermal reaction kettle in a blast drying oven, heat it to 150°C for 0.5h of hydrothermal reaction, and let the resulting hydrothermal reaction system naturally After being cooled to room temperature, the obtained hydrothermal reaction product is vacuum-filtered on a sand core suction filter device (using a water-based fiber filter membrane with a pore size of 0.15 μm); the filtered solid material is placed in a blast drying oven and Dry at 80°C to obtain α-manganese dioxide, denoted as MnO 2 -150-0.5.
Embodiment 2
[0046] Dissolve 2.04g of manganese sulfate monohydrate and 1.27g of potassium permanganate in 80mL of deionized water, stir magnetically for 15min, then add 4mL of hydrochloric acid solution with a mass concentration of 37%, and stir for 5min to obtain a mixed solution; transfer the mixed solution Put it into a 100mL hydrothermal reaction kettle with a stainless steel shell and a polytetrafluoroethylene liner, and place the hydrothermal reaction kettle in a blast drying oven, heat it to 120°C for a hydrothermal reaction for 12 hours, and cool the resulting hydrothermal reaction system naturally After reaching room temperature, the obtained hydrothermal reaction product is vacuum-filtered on a sand core suction filter device (using a water-based fiber filter membrane with a pore size of 0.15 μm); the filtered solid material is placed in a blast drying oven and Dry at 80°C to obtain α-manganese dioxide, denoted as MnO 2 -120-12.
[0047] Carry out performance test and character...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com