Pyridyl phosphamide compound as well as preparation method thereof and application thereof as nickel-cobalt extracting agent
A technology for pyridylphosphoramides and compounds, which is applied in the field of pyridylphosphoramide compounds, can solve the problems of poor selectivity and difficulty in applying nickel-cobalt extraction and separation, and achieve the effects of enhanced selectivity, benefit for large-scale production, and improved extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of intermediate product p-pyridine formaldehyde acetal 2-ethylhexylamine
[0047] In a 250 ml round bottom flask, pyridine-4-carbaldehyde (21.38 g, 0.2 mol), 2-ethylhexylamine (25.90 g, 0.2 mol) and 1.0 g of p-toluenesulfonic acid were dissolved in absolute ethanol (150 mL) , stirred and refluxed in an oil bath at 80°C for 8h, tracked and detected by TLC until the reaction was complete, and added 1.54g of anhydrous K 2 CO 3 , continue to react for 30min, cooled to room temperature, concentrated under reduced pressure, washed with water, extracted three times with 100ml ethyl acetate, collected the organic phase, dried over anhydrous magnesium sulfate, filtered, removed the organic solvent by rotary evaporation in vacuum, and dried in vacuum. Yield: 41.24 g. Yield 94.50%.
Embodiment 2
[0049] Synthesis of extractant α-phosphoramidate (abbreviated as EHPYEP)
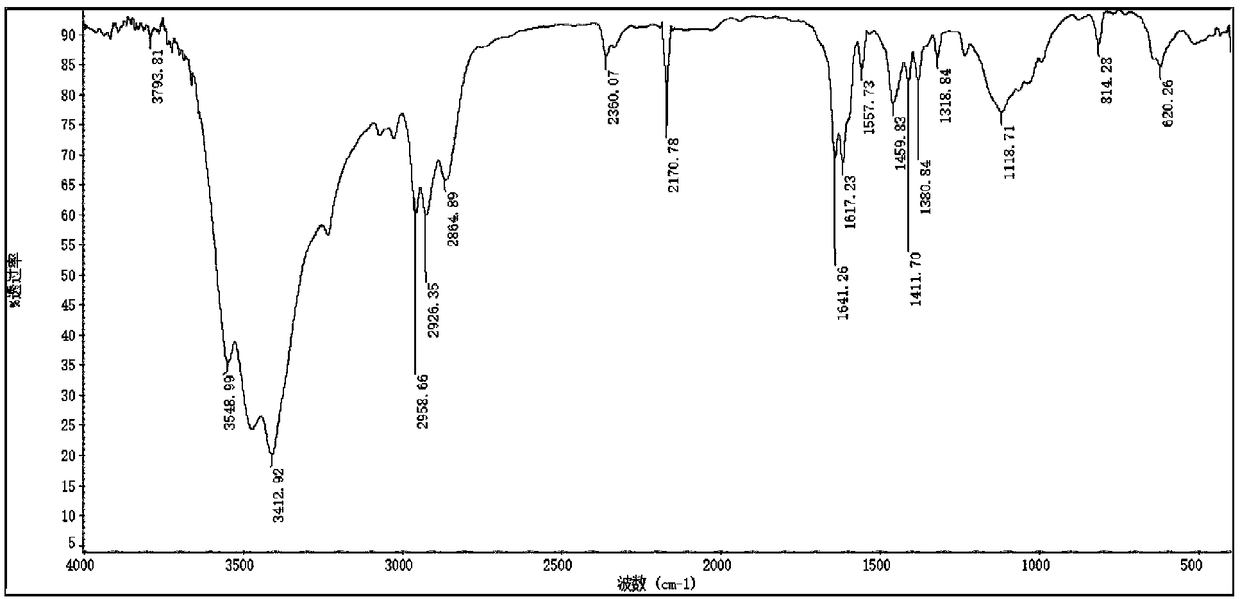
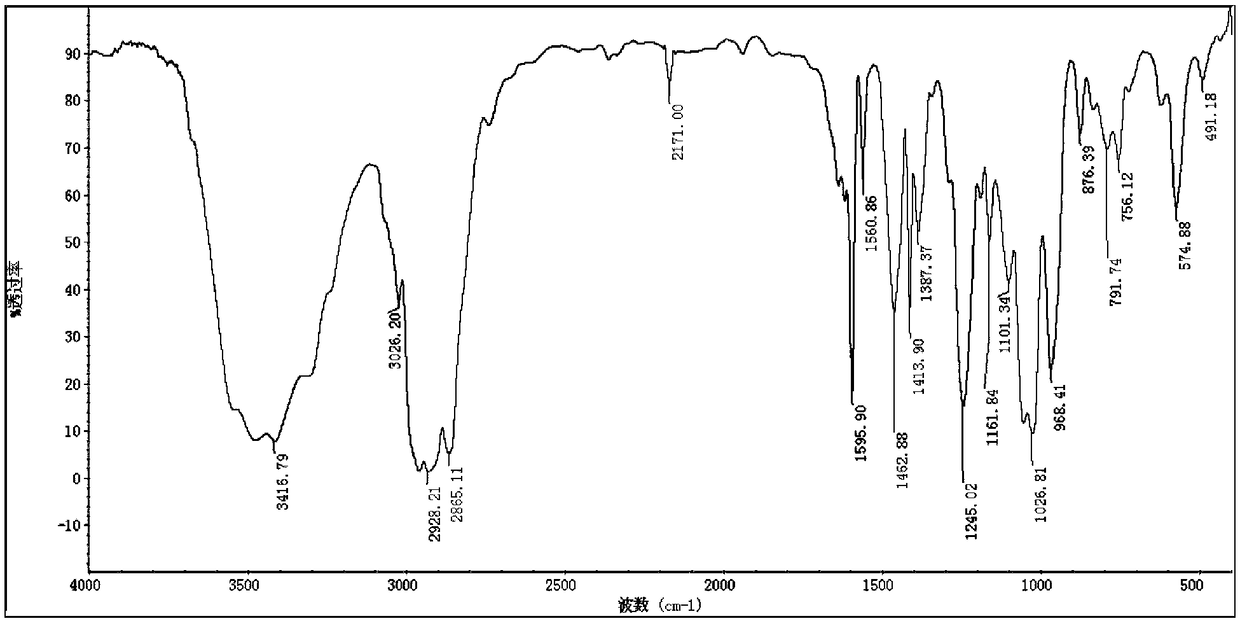
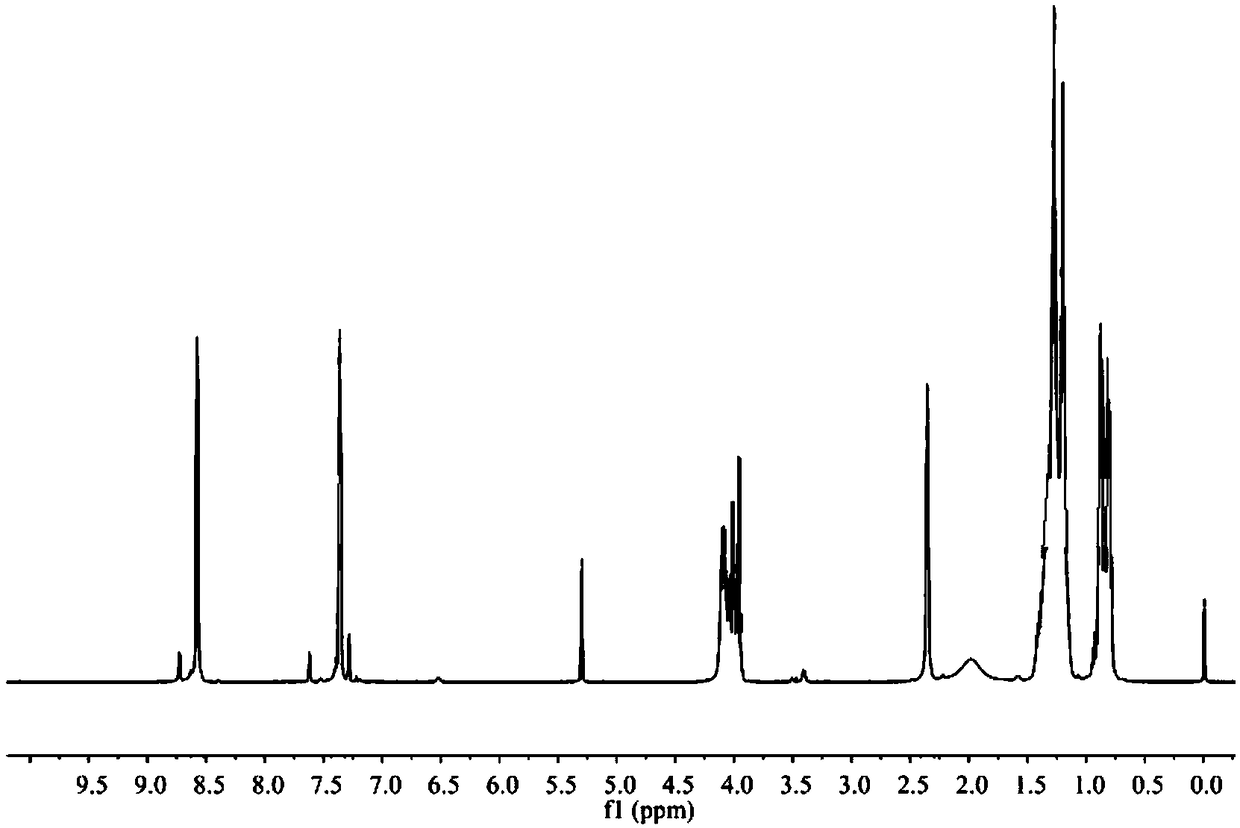
[0050] In a 500ml round bottom flask, the product obtained in the previous step (43.66g, 0.2mol) and diethyl phosphite (27.65g, 0.2mol) were reacted in an oil bath at 80°C for 5h without solvent, followed by TLC to detect whether the reaction was complete , cooled to room temperature, transferred to a 500ml separatory funnel, washed with acid 3 times, washed with alkali 3 times, washed with water until neutral, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, combined organic phases, vacuum rotary evaporation to remove acetic acid Ethyl ester was dried in vacuo overnight to obtain wine red liquid. Yield: 68.63 g. Yield 96.34%. The structure is characterized as follows: IR(KBr)ν / cm -1 : 3417, 3026, 2928, 1596, 1561, 1463, 1387, 1245, 1027, 968, 876, 792, 575; 1 H NMR (500MHz, CDCl 3 )δ H : 8.57(d, J=4.0Hz, 2H), 7.37(dd, J=9.3, 7.5Hz, 2H), 4.14–3.93(m, 5H), 2.35(s, 2H)...
Embodiment 3
[0052] Water phase feed liquid: simulated feed liquid, feed liquid containing Ni 1.187g / L, Co 1.163g / L, Mg 1.309g / L, Mn1.204g / L, pH 5.81;
[0053] Organic phase: use sulfonated kerosene as diluent, extractant is single extractant EHPYEP, concentration setting: 0.10, 0.15, 0.20, 0.,25mol / L.
[0054] Extraction: The ratio between the organic phase and the feed liquid (O / A) was 2:1, the mixing time was 5 minutes, the oscillation frequency was 200r / min, and the extraction was carried out at room temperature. The experimental results are shown in Table 1 below.
[0055] It can be seen from Table 1 that the single extractant EHPYEP has poor ability to extract nickel, cobalt, magnesium and manganese, and almost has no extraction effect.
[0056] The extraction effect of table 1 single extractant EHPYEP
[0057] EHPYEP Concentration
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com