Tumor neoantigen detection method, device and storage medium based on next-generation sequencing
A technology for antigen detection and next-generation sequencing, which is applied in biochemical equipment and methods, microbial measurement/inspection, genomics, etc., and can solve problems such as false positives, false positives in high-quality neoantigen screening, and failure to consider mutant peptides , to achieve the effect of reducing false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] This example uses Yadav, Mahesh, et al. * # * Predicting Immunogenic Tumour Mutational and Exme Sequencing. * # * Nature 515.7528 (2014): 572. Data from Document (hereinafter referred to as Document 1): Mouse Model MC-38 tumor sample and normal sample exterior data, and transcript data; adoption of second-generation tumor new antigen detection method, the tumor new antigen detection, as follows:
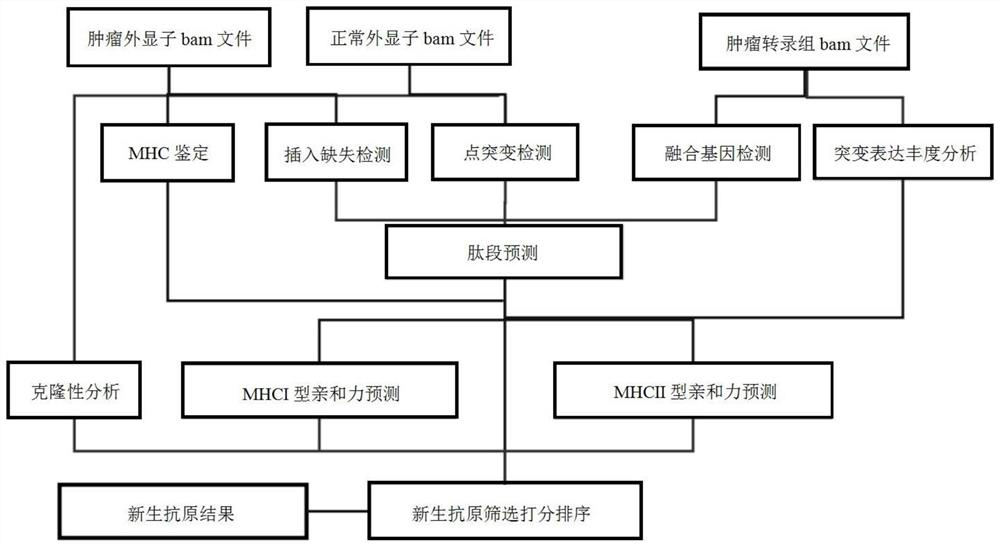
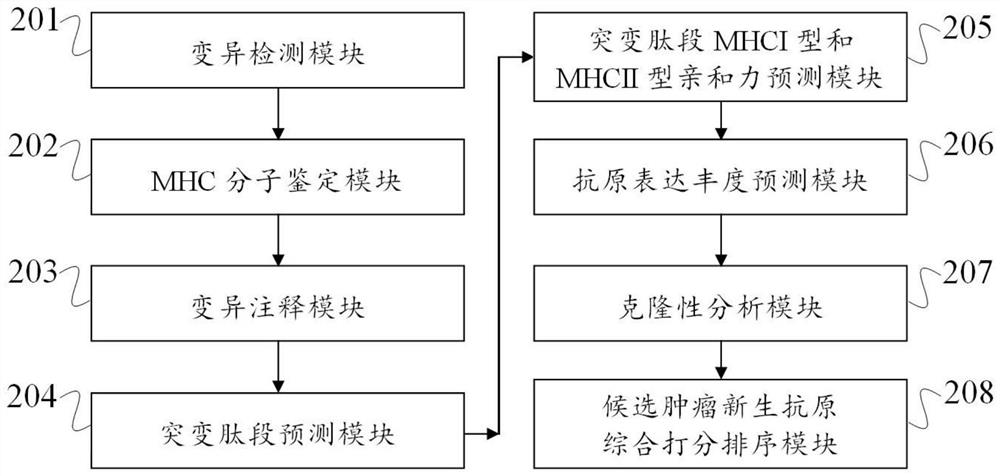
[0096] (1) Variation test
[0097] Insertion and deleion, Indel are detected by Varscan and Mutectide, SINGLE NUCLETIDE VARIANT, SNV. In order to obtain high quality mutations, the intersection of two software is used as high quality candidate mutations. For the detection of the fusion gene, STAR-Fusion is applied to detect comparison RNA BAM format files.
[0098] (2) MHC molecular identification
[0099] In order to examine the type of MHC-I and MHC-II molecules, this example uses Polysolver to detect the HLA molecular type of normal samples and tumor samples. If the HLA molecul...
Embodiment 2
[0134] 利用发表数据ICC24(Sia D,Losic B,Moeini A,et al.Massive parallelsequencing uncovers actionable FGFR2-PPHLN1fusion and ARAF mutations inintrahepatic cholangiocarcinoma.[J].Nature Communications,2015,6:6087-6087.),采用实施例1 Tumor neovascular antigen detection method for new antigen detection. The results showed that 5 antigen peptides identified by HLA were detected, including the high frequency of the ICC, which can be identified by HLA-01, derived from the fusion gene FGFR2-pphln1 of hepatic cholangiocarcinoma. It can be seen that the new tumor new tumor neovascular antigen in cholangiocyte carcinoma is found using the tumor new antigen detection method of Example 1. There is no good treatment for advanced biliary cell carcinoma, the survival rate is low; the new antigen has been obtained by the method of Example 1, and the new type of treatment of biliary cell carcinoma is found, which provides a new solution for the treatment of biliary cell carcinoma. And the way.
Embodiment 3
[0136] Application This method is used for new antigen detection of 288 liver inner bile duct cancer (ICC) samples, 288 liver bile duct cancer samples from the following 4 documents:
[0137] Hiromi Nakamura, Yasuhito Arai1, Yasushi Totoki, et al.genomic spectraof biliary tract can, [j] .nature menetics, 2015, 47 (9): 1003.
[0138] .
[0139] Yuchen Jiao,Timothy M Pawlik,Robert A Anders,et al.Exome sequencingidentifies frequent inactivating mutations in BAP1,ARID1A and PBRM1 inintrahepatic cholangiocarcinomas.[J].Nature Genetics,2013,45(12):1470-U93.
[0140] Sikiş sikiş sikiş sikiş sikiş sikiş 05:
[0141] The analysis results of 18,813 non-synonymous mutations of 288 ICC samples show that each ICC sample can find 22.8 mutant antigen peptides recognized by high-frequency HLA genotypes in the population, with 62% of Clonalmutation. Note These samples can be treated in patients when there is no suitable targeted drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com