An ultra-high antibacterial performance austenitic stainless steel for medical implant stents
An austenitic stainless steel and performance technology, applied in the field of ultra-high antibacterial performance austenitic stainless steel materials and their preparation, can solve the problems of human health impact, reduced service life time, inability to apply, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
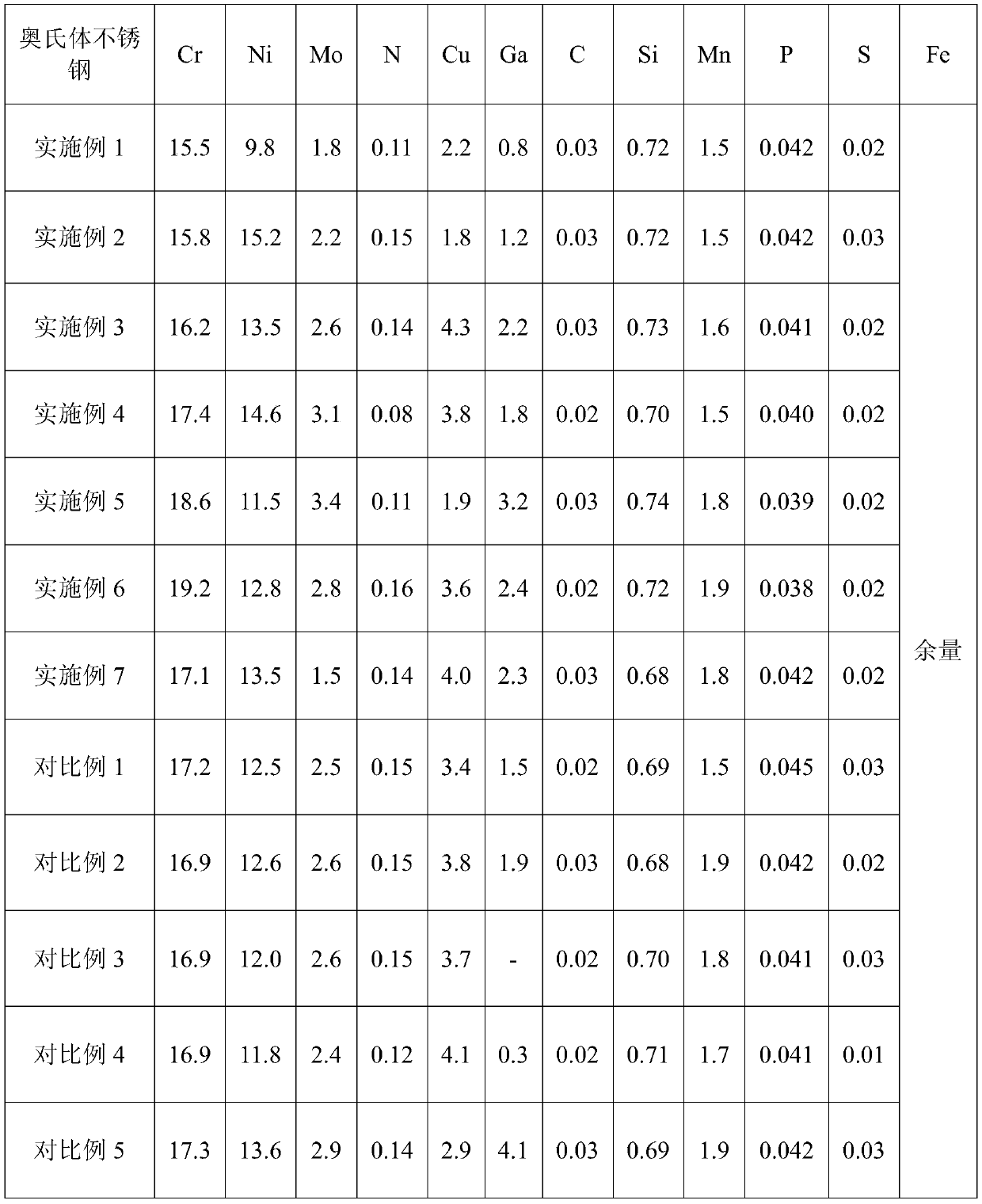
[0026]According to the chemical composition range set by the ultra-high antibacterial performance austenitic stainless steel material, the present invention adopts 15 kilograms of vacuum induction furnace to smelt the embodiment and comparative example to forge 10 kilograms of ultra-high antibacterial performance austenitic stainless steel respectively, and its chemical composition is shown in Table 1 .
[0027] The main chemical composition (wt.%) of the austenitic stainless steel of table 1 embodiment and comparative example
[0028]
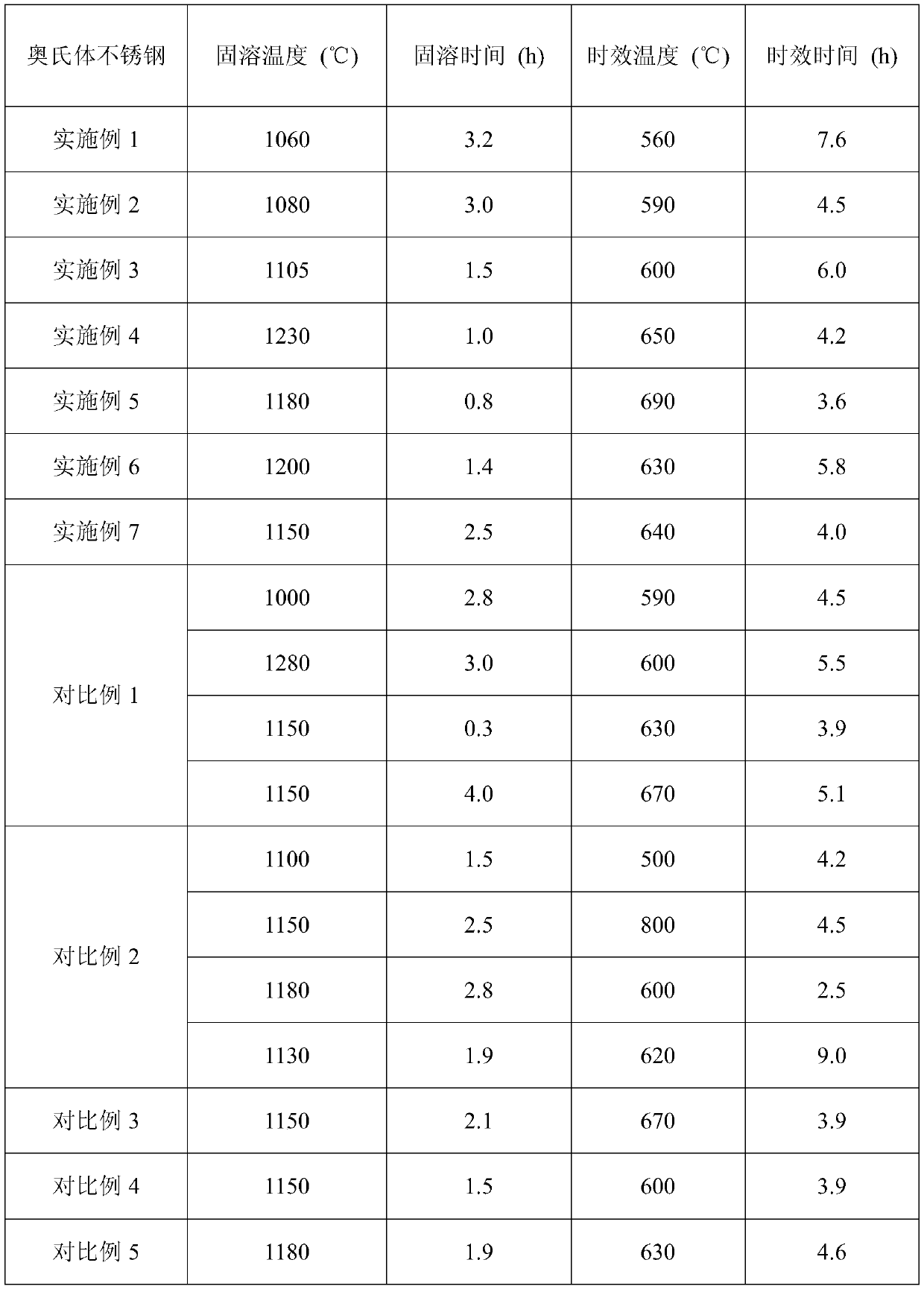
[0029] According to the parameter range of the heat treatment method set by the ultra-high antibacterial performance austenitic stainless steel of the present invention, the detailed parameters of the solid solution and aging heat treatment formulated are shown in Table 2.
[0030] The heat treatment process parameter of table 2 embodiment and comparative example
[0031]
[0032] 1. In vitro antibacterial performance test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com