Crosslinkable naphthal-diimide-based all-polymer solar cell receptor material and preparation method and application thereof
A solar cell and naphthalene diimide technology, which is applied in the fields of electric solid-state devices, semiconductor/solid-state device manufacturing, circuits, etc., can solve the problems of hindering charge separation and transmission, efficiency attenuation, and the service life gap of all-polymer solar cells. , achieve high photoelectric conversion efficiency, improve thermal stability, and improve thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
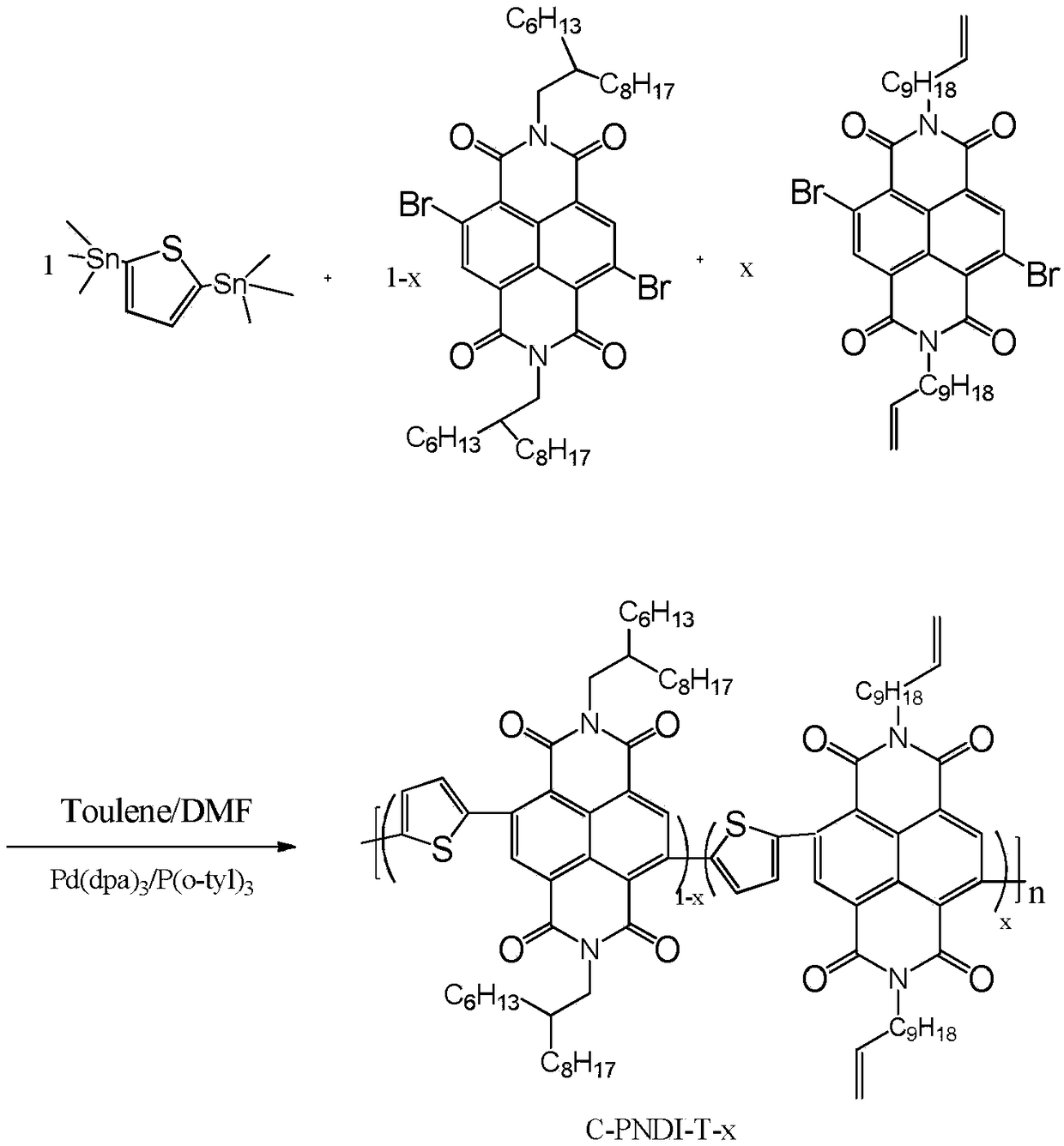
[0037] Synthesis of Polymer C-PNDI-T-0.05
[0038] The chemical reaction flow chart of the present embodiment is as figure 1 Shown, concrete reaction steps and reaction conditions are as follows:
[0039] Under nitrogen protection, 0.01045 mmol monomer A (4,9-dibromo-2,7-bis(2-hexyldecyl)-benzo[lmn][3,8]o-phenanthroline-1, 3,6,8-tetranitro), 0.0055mmol monomer B (4,9-dibromo-2,7-di(undecenyl)-benzo[lmn][3,8]o-diazepine After mixing phenanthrene-1,3,6,8-tetranitro and 0.11mmol monomer C (2,5-bis(trimethylstannyl)thiophene), add 5mol% catalyst tris(dimethoxyphene) in sequence Benzylacetone) Dipalladium (Pd 2 (dba) 3 ) and 10mol% ligand tris(o-methylphenyl)phosphine (P(o-tyl) 3) were mixed and dissolved in 3 mL of a mixed solvent of toluene and N,N dimethylformamide (5:1). Then allow the reaction solution to stir and reflux at 110°C for 48 hours, then add 0.11 mmol of phenylboronic acid to cap it, and continue the reaction for 6 hours; stop responding. After the reaction ...
Embodiment 2
[0052] Synthesis of Polymer C-PNDI-T-0.03
[0053] The chemical reaction flow chart of the present embodiment is as figure 1 Shown, concrete reaction steps and reaction conditions are as follows:
[0054] Under nitrogen protection, 0.1012 mmol of monomer A (4,9-dibromo-2,7-bis(2-hexyldecyl)-benzo[lmn][3,8]o-phenanthroline-1, 3,6,8-tetranitro), 0.0088mmol monomer B (4,9-dibromo-2,7-di(undecenyl)-benzo[lmn][3,8]o-diazepine After mixing phenanthrene-1,3,6,8-tetranitro and 0.11 mmol monomer C (2,5-bis(trimethylstannyl)thiophene), add 5 mol% catalyst tris(dibenzyl Dipalladium (Pd 2 (dba) 3 ) and 10mol% ligand tris(o-methylphenyl)phosphine (P(o-tyl) 3 ) were mixed and dissolved in 3mL of toluene and N,N dimethylformamide (5:1) mixed solvent; then the reaction solution was stirred and refluxed at 110°C for 48h, then capped with 0.11mmol phenylboronic acid, and the reaction was continued After 7 hours, add 0.3~0.5mL of bromobenzene to block, and then react at 110°C for 4 hours, ...
Embodiment 3
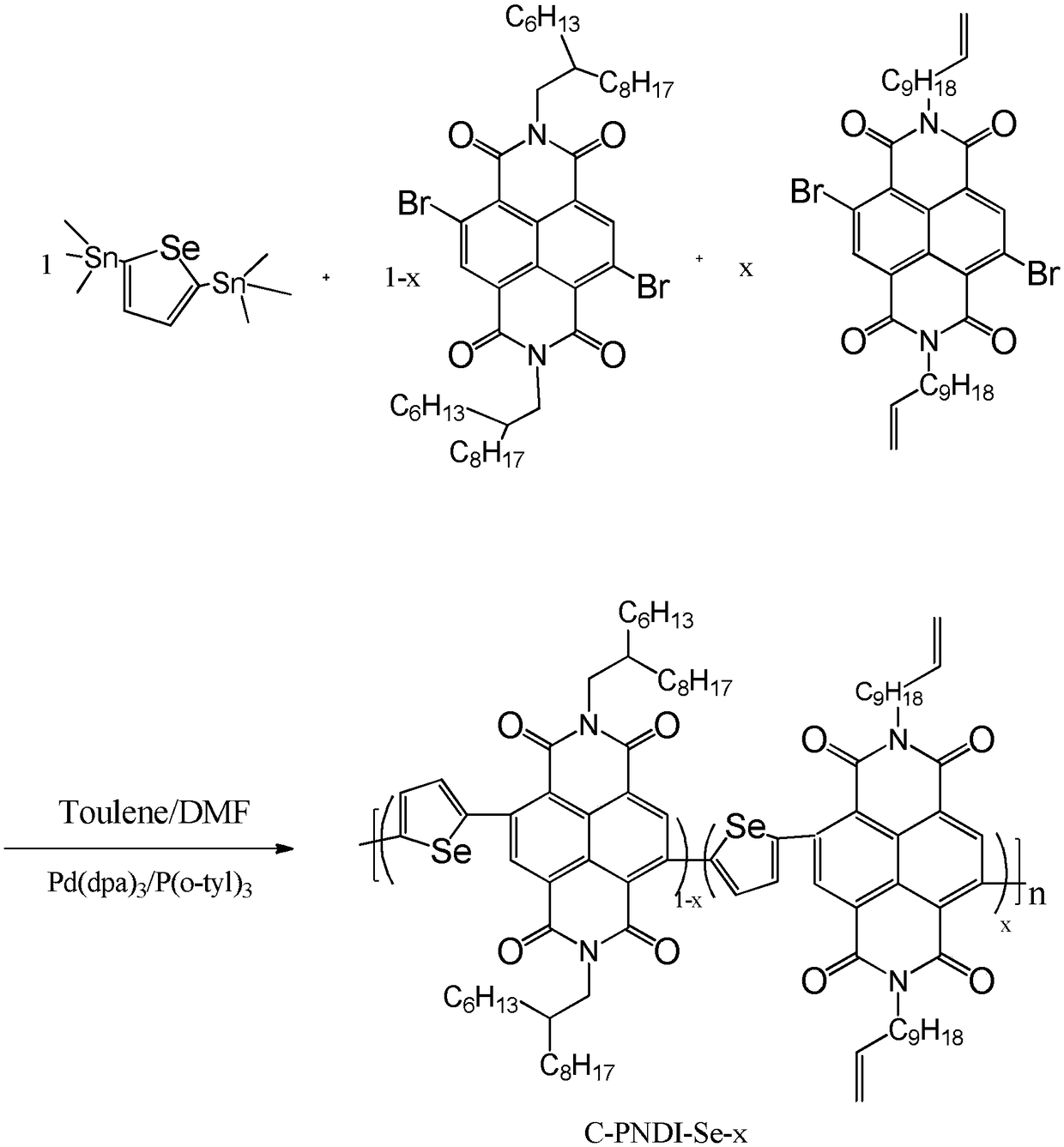
[0061] Synthesis of Polymer C-PNDI-Se-0.05
[0062] The chemical reaction flow chart of the present embodiment is as image 3 Shown, concrete reaction steps and reaction conditions are as follows:
[0063] Under nitrogen protection, 0.1045 mmol of monomer A (4,9-dibromo-2,7-bis(2-hexyldecyl)-benzo[lmn][3,8]phenanthroline-1, 3,6,8-tetranitro), 0.0055mmol monomer B (4,9-dibromo-2,7-di(undecenyl)-benzo[lmn][3,8]o-diazepine After mixing phenanthrene-1,3,6,8-tetranitro and 0.11 mmol monomer C (2,5-bis(trimethylstannyl)selenophene), 5 mol% catalyst tris(di Benzylideneacetone) Dipalladium (Pd 2 (dba) 3 ) and 10mol% ligand tris (o-methylphenyl) phosphine (P (o-tyl) 3) are mixed and dissolved in 3 mL of toluene and N,N dimethylformamide (5:1) mixed solvent; then Stir and reflux the reaction solution at 110°C for 36 hours, then add 0.11 mmol of phenylboronic acid to block, and continue the reaction for 4 hours; then add 0.3 to 0.5 mL of bromobenzene to block, then react at 110°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com