Indoleamine 2,3-dioxygenase inhibitor and application thereof
一种选自、烷基的技术,应用在医药领域,能够解决抑制效力低下等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
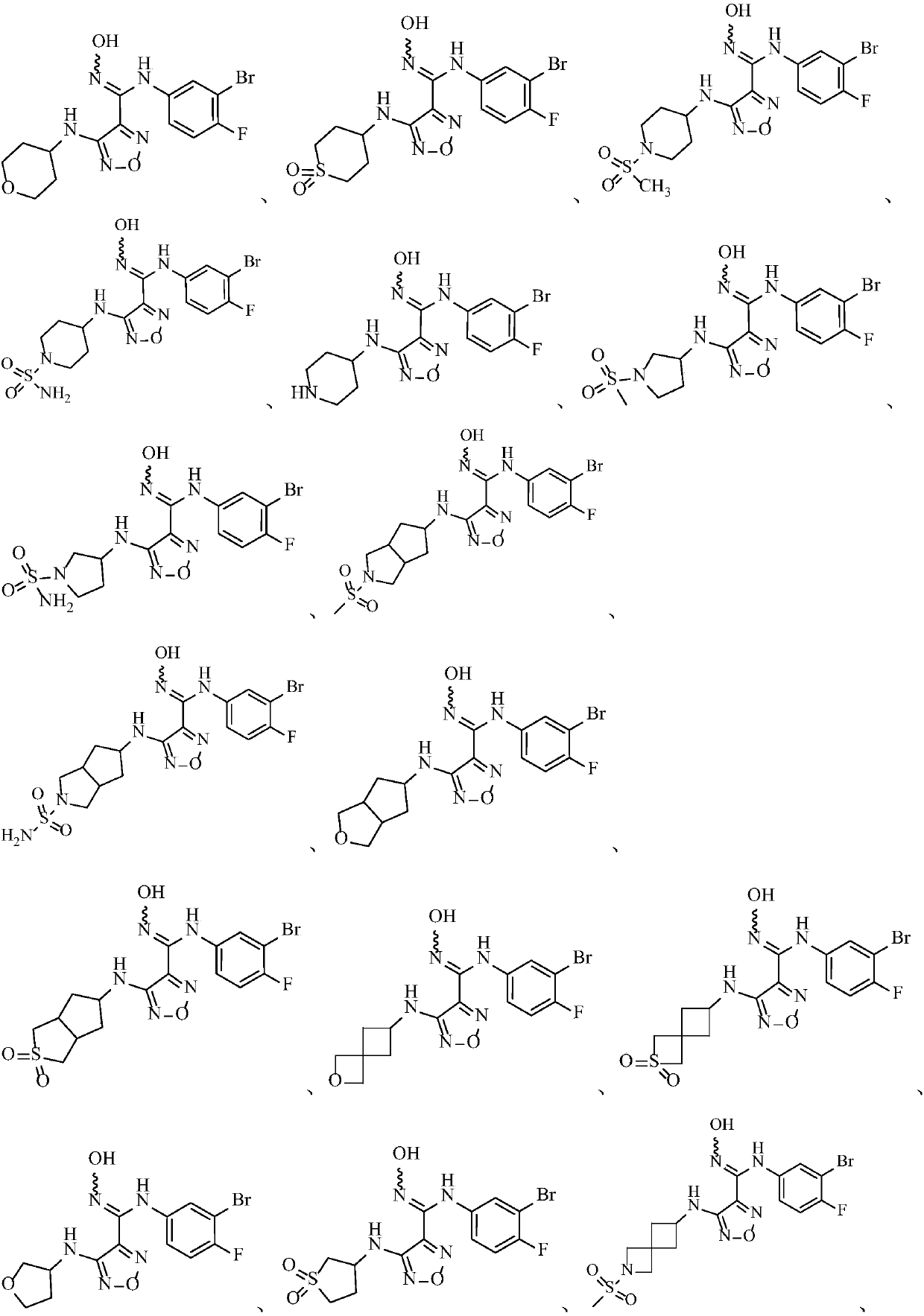
Image
Examples
specific Embodiment approach
[0142] EA: ethyl acetate
[0144] DMA: N,N-Dimethylacetamide
[0145] THF: Tetrahydrofuran
[0146] DCM: dichloromethane
[0147] TEA: Triethanolamine
[0148] TFA: Trifluoroacetic acid
preparation example 1
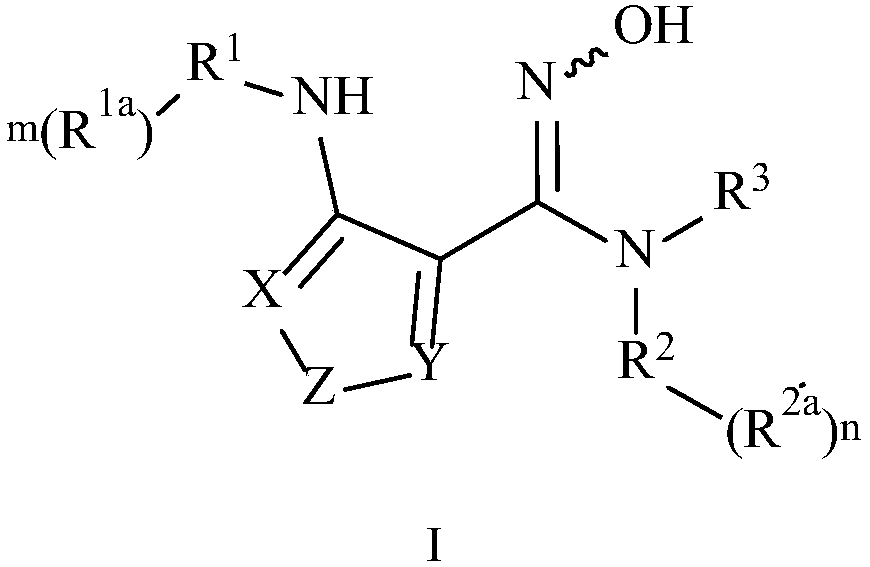
[0149] Preparation Example 1 Synthesis of 4-amino-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboxamidine
[0150]
[0151] Step 1: Synthesis of 4-amino-N'-hydroxy-1,2,5-oxadiazole-3-carboxamidine
[0152]
[0153] Add the raw material malononitrile (50.0g, 0.76mol, 1.0eq) into a four-neck flask, heat to dissolve (about 50°C), add water (0.5L), and put in an ice-water bath at about 10°C, add sodium nitrite (57.72 g, 0.83mol, 1.1eq), after the addition was completed, hydrochloric acid (6N, 8.5mL) was added dropwise below 10°C, after the drop was completed, the mixture was stirred in an ice-water bath for 0.5h until the temperature remained constant, and the above reaction solution was designated as A. H 2 NOH·HCl (158.4g, 2.28mol, 3.0eq) was dissolved in water (255mL), and a solution of potassium hydroxide (127.9g, 2.28mol, 3.0eq) in water (255mL) was added thereto. ℃) stirred for 10 min, and the above reaction solution was denoted as B. Cool the reactio...
preparation example 2
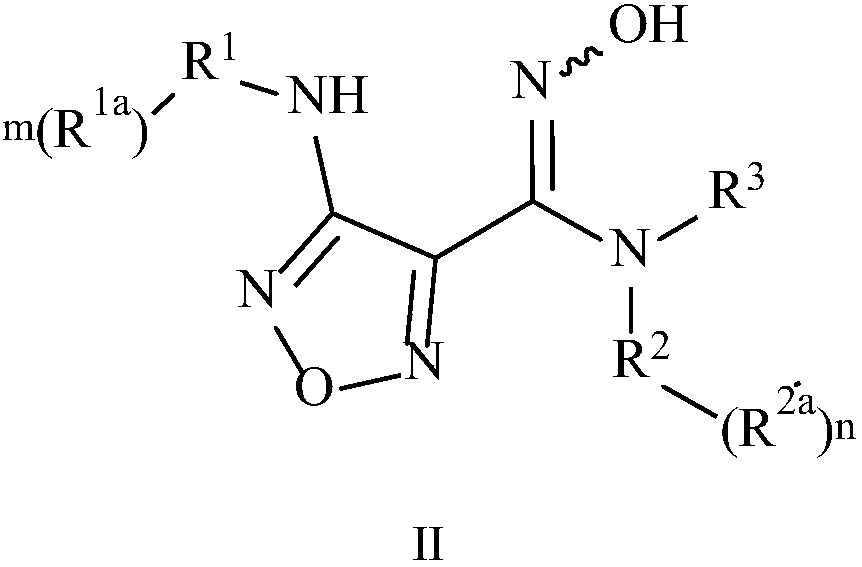
[0163] Preparation 2 4-(3-bromo-4-fluorophenyl)-3-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazole- Synthesis of 5(4H)-ketone
[0164]
[0165] Step 1: 3-(4-Amino-1,2,5-oxadiazol-3-yl)-4-(3-bromo4-fluorophenyl)-1,2,4-oxadiazol-5( Synthesis of 4H)-ketone
[0166]
[0167] 4-Amino-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboxamidine (5.0g, 15.8mmol, 1.0eq) was dissolved in Add carbonyldiimidazole (3.85g, 23.7mmol, 1.5eq) to ethyl acetate (65mL), heat the reaction at 60°C for 0.5h, monitor the completion of the reaction by TLC, cool the reaction solution to room temperature, wash with 1mol / L hydrochloric acid (65mL× 2), combine the organic phases, separate the organic phases, concentrate to dryness, the crude product is subjected to beating and suction filtration with methyl tert-butyl ether to obtain 3-(4-amino-1,2,5-oxadiazol-3-yl) -4-(3-Bromo-4-fluorophenyl)-1,2,4-oxadiazol-5(4H)-one (3.53 g, yield: 65%).
[0168] Step 2: 4-(3-Bromo-4-fluorophenyl)-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com