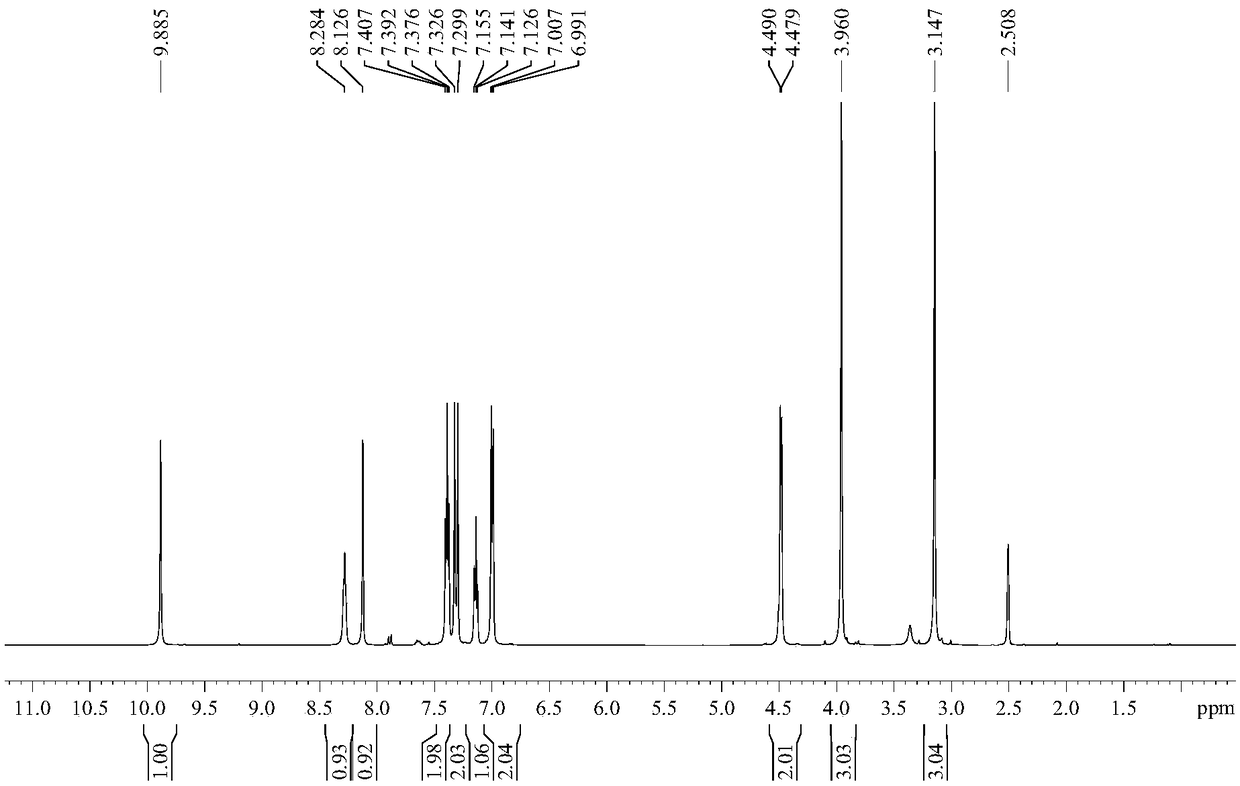
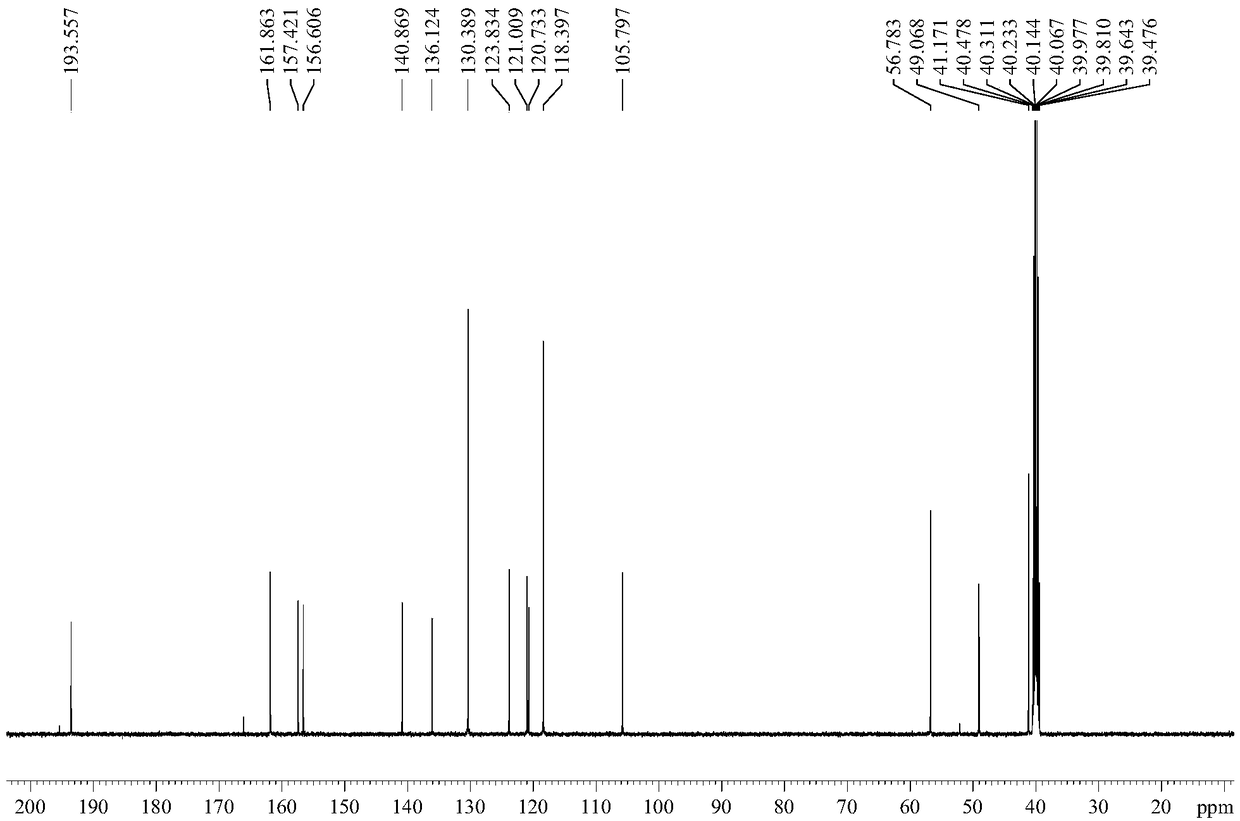
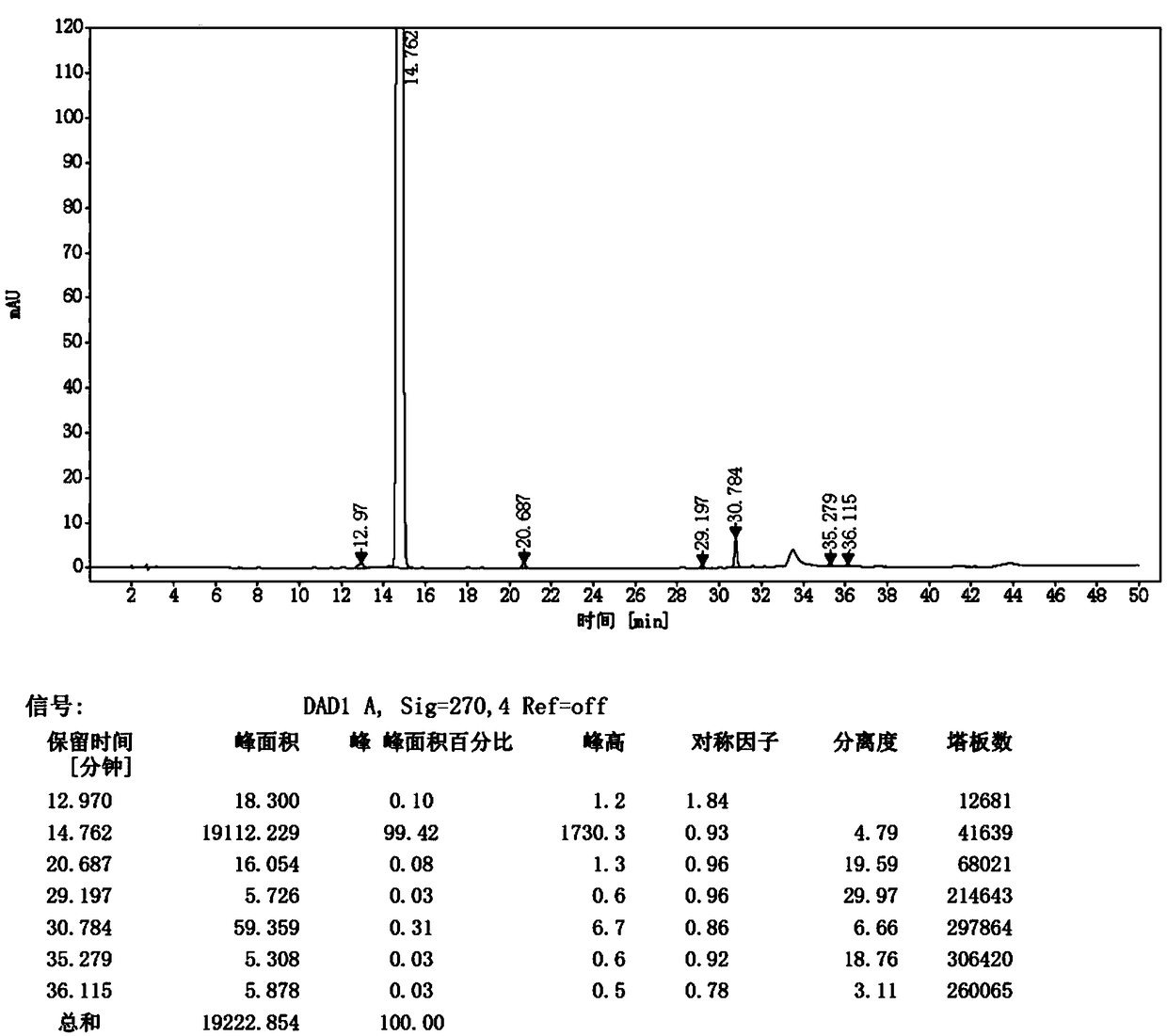
Preparation method of Iguratimod formylation intermediates
A technology of Aylamod formyl and intermediates, which is applied in the field of chemical drug synthesis, can solve the problems of slow reaction rate, many impurities, and low yield, and achieve the effects of fast reaction speed, high yield, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a method for preparing an iguratimod formylation intermediate, comprising the steps of:
[0042] Reaction of formic acid, sodium formate and pivaloyl chloride to obtain a mixed anhydride, adding compound I for carbamoylation reaction to obtain the formylation intermediate of iguratimod;
[0043] Wherein, the structural formula of the compound I is The structural formula of the iguratimod formylation intermediate is
[0044] The invention adopts formic acid and pivaloyl chloride to prepare mixed acid anhydride, because the formic acid is dissolved in acetone, the reaction with pivaloyl chloride is a homogeneous reaction, the reaction speed is fast, and the conversion rate is high. Moreover, formic acid maintains the relatively acidic environment of the system, avoids the side reaction of the self-condensation reaction of the α-aminoketone compound in the raw material under alkaline conditions to generate piperazine compounds, improves the yield...
Embodiment 1
[0068] A preparation method for an iguratimod formylation intermediate, comprising the steps of:
[0069] (A) Add 1.43kg of formic acid and 2.64kg of sodium formate into 30L of acetone, stir at 18-20°C, and after the dispersion is uniform, add 3.74kg of pivaloyl chloride, stir and react for 1 hour to obtain a system containing mixed anhydrides;
[0070] (B) Add 12.02 kg of 2-amino-1-(2-methoxy-4-methanesulfonamido-5-phenoxy)phenylethanone hydrochloride to the reaction system obtained in step (A) (Compound I) and 2.64kg of sodium formate, the temperature is controlled at 10-20°C, stirred for 3 hours, and the reaction is terminated;
[0071] (C) Reduce the temperature of the system obtained in step (B) by 10±5°C, and slowly add water under stirring conditions, keeping the temperature during the water addition process not exceeding 20°C, after adding water, control the temperature to 15±5°C, and stir After 1h, filter, wash the filter cake with water, and collect the filter cake;...
Embodiment 2
[0073] A preparation method for an iguratimod formylation intermediate, comprising the steps of:
[0074] (A) Add 2.30kg of formic acid and 5.10kg of sodium formate into 48L of acetone, stir at 18-20°C, after the dispersion is uniform, add 9.04kg of pivaloyl chloride, stir for 1 hour, and obtain a system containing mixed anhydride;
[0075] (B) Add 12.02 kg of 2-amino-1-(2-methoxy-4-methanesulfonamido-5-phenoxy)phenylethanone hydrochloride to the reaction system obtained in step (A) (Compound I) and 5.10kg of sodium formate, the temperature is controlled at 10-20°C, stirred and reacted for 3h, and the reaction is terminated;
[0076] (C) Reduce the temperature of the system obtained in step (B) by 10±5°C, and slowly add water under stirring conditions, keeping the temperature during the water addition process not exceeding 20°C, after adding water, control the temperature to 15±5°C, and stir After 1h, filter, wash the filter cake with water, and collect the filter cake; heat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com