Preparation method of intermediate of tyrosine kinase inhibitor
The technology of tyrosine kinase and inhibitor is applied in the field of preparation of intermediates, which can solve the problems of many impurities, difficult purification and low purity of products, and achieve the effects of simplifying post-processing, improving purity and yield, and reducing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
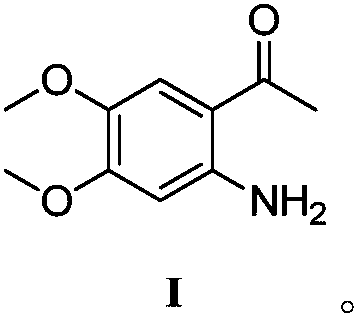
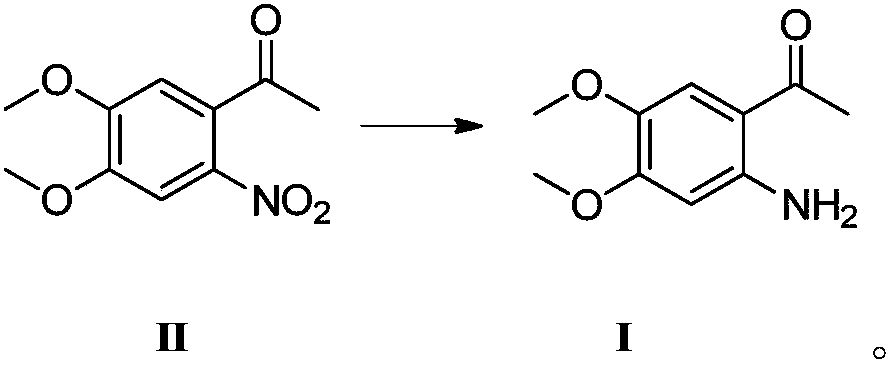
[0023] Put 3.40g (0.01510mol) of compound II in a 250ml four-necked flask, and add a mixed solution of ethyl acetate and water (34ml / 20ml). At room temperature, add 2.10g (0.03925mol, 2.6eq) of ammonium chloride and 3.67g (0.06553mol, 4.34eq) of reduced iron powder to the stirred reaction flask, then heat the reaction system to 40°C and stir for 3h . The progress of the reaction was checked by TLC. After the substrate is completely reacted, the excess iron powder is filtered out. The filter cake was rinsed three times with 15 ml of ethyl acetate. The organic layer was separated from the filtrate, washed with saturated brine, and concentrated under reduced pressure to obtain 2.53 g of Compound I, yield: 86%, HPLC purity: 99.7%.
Embodiment 2
[0025] Put 3.40g of compound II in a 250ml four-necked flask, and add a mixed solution of ethyl acetate and water (34ml / 20ml). At room temperature, 3.55 g (0.06644 mol, 4.4 eq) of ammonium chloride and 5.92 g (0.1057 mol, 7 eq) of reduced iron powder were sequentially added to the stirred reaction flask, and then the reaction system was heated to 20° C. and stirred for 4 hours. The progress of the reaction was checked by TLC. After the substrate is completely reacted, the excess iron powder is filtered out. The filter cake was rinsed three times with 15 ml of ethyl acetate. The organic layer was separated from the filtrate, washed with saturated brine, and concentrated under reduced pressure to obtain 2.79 g of compound I, yield: 94.7%, HPLC purity: 99.2%.
Embodiment 3
[0027] Put 3.40g (0.01510mol) of compound II in a 250ml four-necked flask, and add a mixed solution of ethyl acetate and water (34ml / 20ml). At room temperature, add 1.61g (0.0302mol, 2.0eq) of ammonium chloride and 2.54g (0.0453mol, 3.0eq) of reduced iron powder to the stirred reaction flask, then heat the reaction system to 30°C and stir for 3h . The progress of the reaction was checked by TLC. After the substrate is completely reacted, the excess iron powder is filtered out. The filter cake was rinsed three times with 15 ml of ethyl acetate. The organic layer was separated from the filtrate, washed with saturated brine, and concentrated under reduced pressure to obtain 2.65 g of Compound I, yield: 90%, HPLC purity: 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com