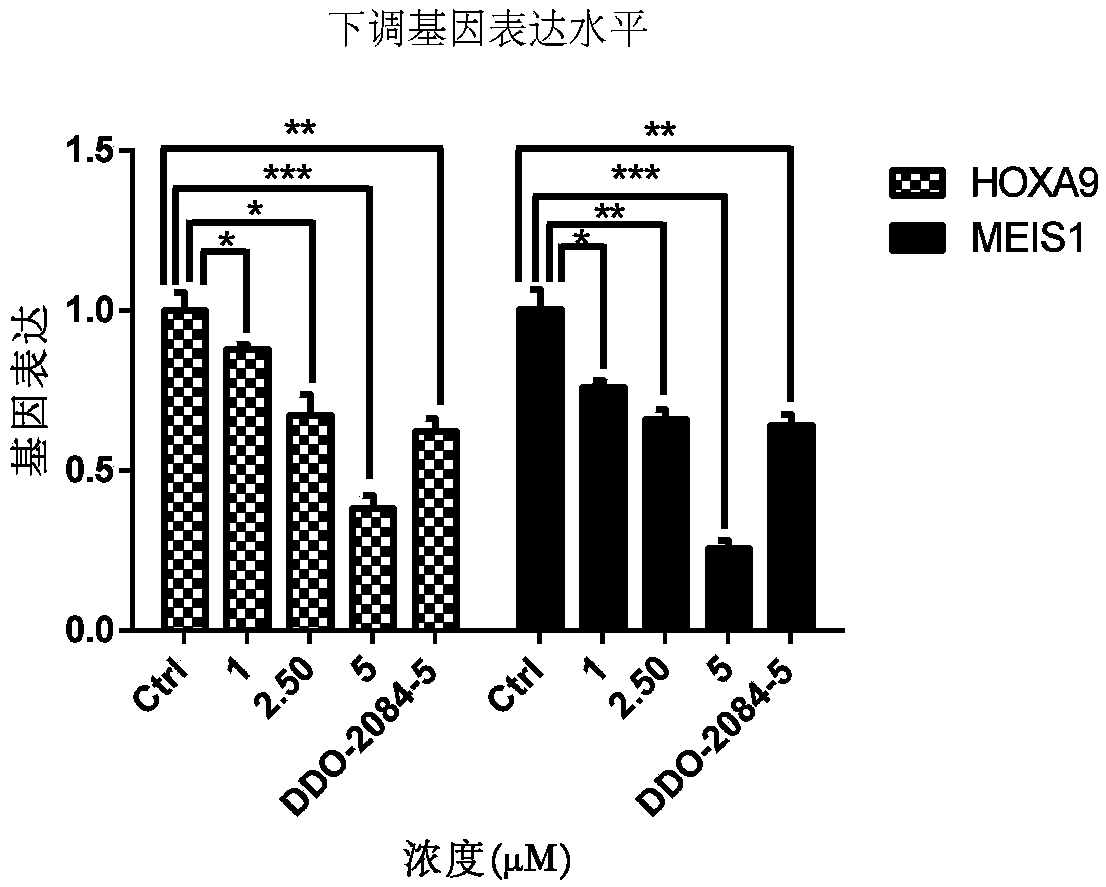
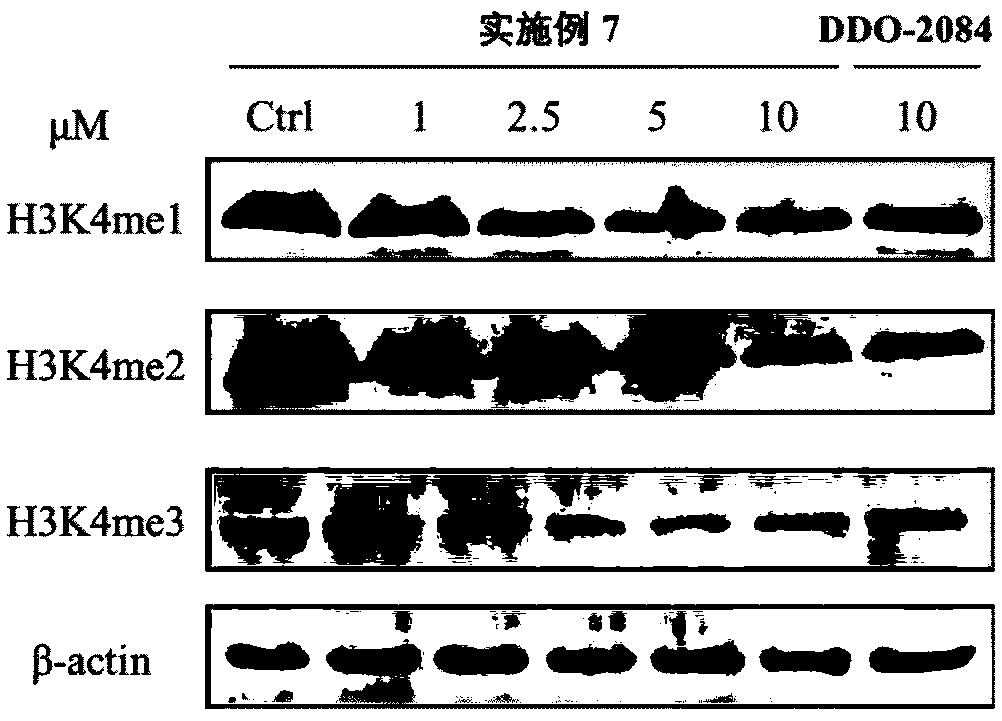
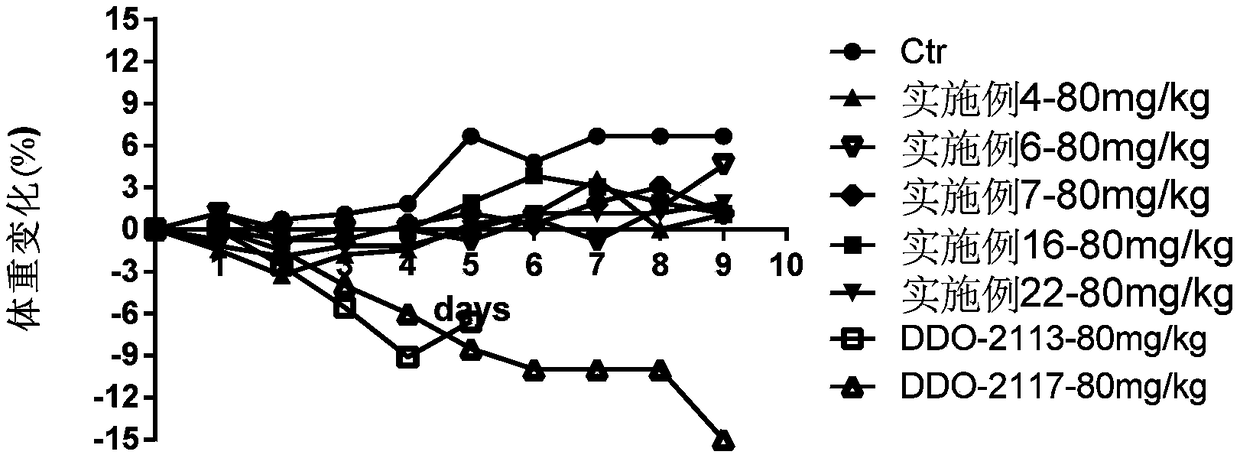
Phenyl triazole MLL1-WDR5 protein-protein interaction inhibitor
A phenyl, C1-C4 technology, applied in the field of medicinal chemistry, can solve problems such as abnormal Hox gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]
[0059] 1-(3-(5-amino-2-chloro-4-fluoro-3-methylbenzamido)-4-(4-methylpiperazin-1-yl)phenyl)-1H-1, Methyl 2,3-triazole-4-carboxylate (1)
[0060] Preparation of 4-(4-methylpiperazin-1-yl)-3-nitroaniline (IIb):
[0061] Compound 4-fluoro-3-nitroaniline (II) (6 g, 38.4 mmol) was dissolved in 50 mL of acetonitrile, N-methylpiperazine (5.8 g, 6.3 mL, 57.6 mmol) and N,N-diiso Propylethylamine (9.5mL, 57.6mmol) was then heated to reflux for 12h. After spin-dried, the crude product was separated and purified by silica gel column chromatography (dichloromethane:methanol=20:1) to obtain a reddish-brown solid (8.9 g, 97.8%). 1 H NMR (300MHz, DMSO-d 6 )δ7.06(d, J=8.6Hz, 1H), 6.76(s, 1H), 6.69(d, J=8.5Hz, 1H), 5.34(s, 2H), 2.70(t, J=4.4Hz, 4H),2.27(br s,4H),2.09(s,3H).m / z(EI-MS):259.1[M+Na] + .
[0062] Preparation of 1-(4-azido-2-nitrophenyl)-4-methylpiperazine (Ia):
[0063] Compound 4-(4-methylpiperazin-1-yl)-3-nitroaniline (IIb) (4.0g, 17.0mmol) was dissolved in 100m...
Embodiment 2
[0071]
[0072] 1-(3-(5-amino-2-chloro-4-fluoro-3-methylbenzamido)-4-(4-methylpiperazin-1-yl)phenyl)-1H-1, Preparation of 2,3-triazole-4-carboxylic acid (2):
[0073] Compound 1-(3-(5-amino-2-chloro-4-fluoro-3-methylbenzamido)-4-(4-methylpiperazin-1-yl)phenyl)-1H- Methyl 1,2,3-triazole-4-carboxylate (1) (2.3g, 4.6mmol) was dissolved in THF, lithium hydroxide solution (1M, 15mL) was added, stirred at room temperature for 8h, then spin-dried to remove THF, Then it was acidified with 2M hydrochloric acid to give a white solid (1.7g, 80.4%). 1 HNMR (300 MHz, DMSO-d 6 )δ9.52-9.45(m,2H),8.69(s,1H),7.73(dd,J=8.7,2.7Hz,1H),7.46(d,J=8.6Hz,1H),6.92(d,J =9.2Hz,1H),5.53(s,2H),3.92(s,3H),3.00-2.90(m,4H),2.50(br s,4H), 2.28(d,J=2.6Hz,3H), 2.24(s,3H).(EI-MS):488.9[M+H] + .
Embodiment 3
[0075]
[0076] 1-(3-(5-amino-2-chloro-4-fluoro-3-methylbenzamido)-4-(4-methylpiperazin-1-yl)phenyl)-N,N- Preparation of dimethyl-1H-1,2,3 triazole-4-carboxamide (3):
[0077] Compound 1-(3-(5-amino-2-chloro-4-fluoro-3-methylbenzamido)-4-(4-methylpiperazin-1-yl)phenyl)-1H- 1,2,3-triazole-4-carboxylic acid (2) (0.18g, 0.36mmol) was dissolved in 10mL of DMF, added BOP (0.32g, 0.72mmol), triethylamine (0.10mL, 0.72mmol) and After dimethylaminohydrochloride (58.7mg, 0.72mmol), stir at room temperature for 4h. Then the reaction solution was diluted by adding 50 mL of ethyl acetate, washed with saturated sodium chloride solution to remove DMF, took the organic phase and dried it over anhydrous sodium sulfate, and spin-dried the organic solvent to obtain the crude product, which was separated and purified by silica gel column chromatography (dichloromethane:methanol=50 : 1) to obtain off-white solid. Yield 78.4%. 1 H NMR (300MHz, DMSO-d 6 )δ9.59(s,1H),9.18(s,1H),8.63(d,J=2.5H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com