Siniperca chuatsi IFN-alpha 3 (interferon alpha-3) gene, recombinant protein, preparation method and application thereof
A technology of recombinant protein and squid mandarin fish, which is applied in the fields of botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems such as difficult to use immunization methods, so as to improve anti-virus ability, reduce mortality, express The effect of volume increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Cloning of the full-length cDNA sequence of the mandarin fish IFN-α3 in embodiment 1:
[0048] 1. Synthesis of primers
[0049] The partial cDNA sequence of the IFN-α3 gene of Siniperca sinensis was searched from the sequencing results of the transcriptome of Siniperca sinensis. The cDNA sequence is shown in SEQ ID NO: 1, and the expressed IFN-α3 recombinant protein is shown in SEQ ID NO: 2 Show.
[0050] 2. Extraction of sample RNA
[0051] The RNA in the collected Siniperca sinensis spleen was extracted by Trizol method. Use NANODROP 2000 (Thermoscientific, USA) ultra-micro ultraviolet spectrophotometer to detect the quality and concentration of RNA, and perform agarose electrophoresis to check the integrity of the extracted RNA. If the quality of the extracted RNA is not up to standard, repeat this step to extract again until a qualified RNA sample is obtained.
[0052] 3. Amplification of Siniperca sinensis IFN-α3 5' end sequence
[0053] (1) Use SUPERSCRIPT II...
Embodiment 2
[0071] The construction of embodiment 2 prokaryotic expression vector
[0072] 1. Molecular cloning of IFN-α3 of Siniperca sinensis
[0073] (1) Design primers IFN-α3-EcoRI-F and IFN-α3-XhoI-R with restriction sites according to the cDNA sequence of IFN-α3 after removing the signal peptide:
[0074] IFN-α3-EcoRI-F: 5'-TCCGAATTCTGTGATTGGCTCA-3'
[0075] IFN-α3-XhoI-R: 5'-GCTCGAGTCAGTGTTGGTGA-3'.
[0076] (2) The PCR reaction system is as follows:
[0077]
[0078] (3) The PCR reaction procedure is as follows:
[0079]
[0080] 2. The target fragment is connected to the T carrier: observe the target band by gel electrophoresis of the product obtained by PCR, and then recover it with a gel kit and connect it to the T carrier.
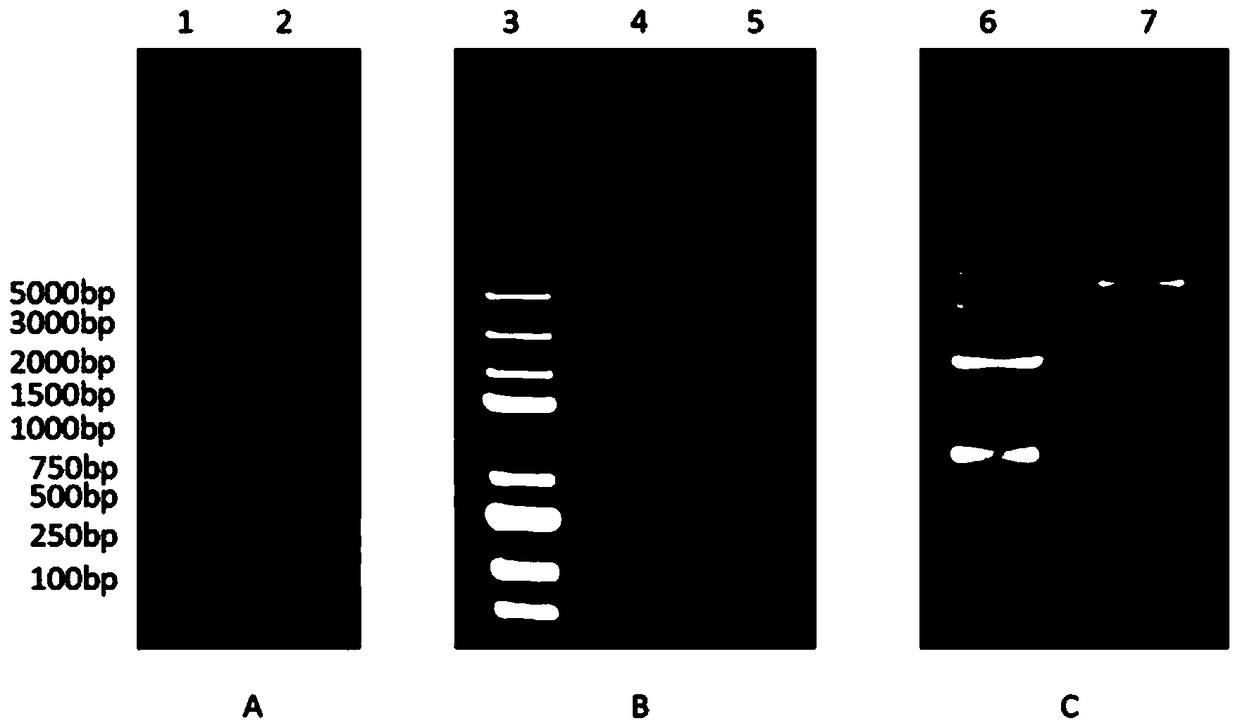
[0081] During the above experiment, figure 1 Swimming lane 2 of A shows the IFN-α3 ORF sequence obtained by PCR, 496bp; after it is connected with pMD19T and sequenced correctly, it is double digested with EcoR I and Xho I, and the target fragme...
Embodiment 3
[0084] Example 3 Prokaryotic expression of recombinant IFN-α3 of Siniperca sinensis
[0085] 1. Construction of recombinant expression plasmids: double enzyme digestion of the pMD19-T-IFN-α3 plasmid and the expression vector pET32a(+) plasmid, the enzyme digestion system is as follows:
[0086]
[0087] 2. Agarose gel electrophoresis, and tap the gel to recover the target fragment after digestion, such as figure 1 As shown in B, the IFN-α3 fragment was ligated with the vector pET32a(+) at a molar ratio of 5:1, and the expression vector was obtained overnight at 4°C.
[0088] 3. Transfer the above-mentioned ligation product into DH5α competent cells, pick positive clones, identify them by bacterial liquid PCR, and select positive clones to extract plasmids.
[0089] 4. Transform the correct recombinant plasmid into the expression strain E.coliBL21(DE3).
[0090] 5. Induction of fusion protein expression
[0091] Pipette 5 μL of pET-32a(+)-IFN-α3 / BL21 bacterial solution an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com