A kind of juniperane sesquiterpene compound and its preparation and application
A technology of sesquiterpenoids and juniperanes, applied in the field of juniperanesesquiterpenoids and their preparation, can solve the problems of biological and human health hazards, serious residues, soil pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
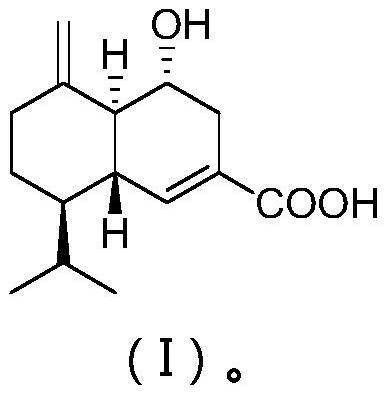
[0022] The structure of the juniperane sesquiterpenoid derived from the epiphytic fungus of seaweed is shown in formula (I).
[0023]
[0024] The compound has the following physicochemical and spectroscopic properties:
[0025] Colorless oil; specific rotation [α] 22 D -164 (c0.22, MeOH); H NMR spectrum (solvent is deuterated dimethyl sulfoxide) δ H 1.80(m), 3.78(td, 9.5, 6.0), 2.67(dd, 17.4, 5.5), 1.97(m), 6.78(br s), 1.81(m), 1.35(br t, 11.1), 1.77(m ), 1.09(qd, 12.7, 4.4), 2.30(br d, 11.5), 1.94(m), 2.12(m), 0.73(d, 6.9), 0.91(d, 6.9), 4.95(br s), 4.79 (br s); C NMR spectrum (solvent is deuterated dimethyl sulfoxide) δ C 50.5(CH), 65.9(CH), 34.8(CH 2 ), 130.5(C), 135.6(CH), 45.3(CH), 45.9(CH), 26.6(CH 2 ), 36.5 (CH 2 ), 148.4(C), 168.9(C), 26.5(CH), 14.9(CH 3 ), 21.2 (CH 3 ), 106.2 (CH 2 ); high-resolution mass spectrum [M]+m / z 250.1568, calculated value 250.1569.
Embodiment 2
[0027] The preparation method of the juniperane sesquiterpenes shown in formula (I):
[0028] Take Trichoderma virens (Trichoderma virens) Y13-3 bacterial classification that grows well on the plate, cut into small pieces and inoculate in potato dextrose liquid medium, put 300 ml of medium in each 1 liter Erlenmeyer flask, 200 bottles in total, room temperature Static fermentation for 30 days, then extracted three times with ethyl acetate, concentrated under reduced pressure to obtain 27.2 grams of crude extract after concentration.
[0029] The potato glucose liquid medium consists of 500 milliliters of boiled juice containing 100 grams of potatoes per liter, 20 grams of glucose, 5 grams of peptone, 5 grams of yeast extract powder, and 500 milliliters of aged sea water.
[0030] Trichoderma virens (Trichoderma virens) Y13-3 strain was preserved in the China Center for Type Culture Collection CCTCC on January 10, 2018, address: Wuhan University, China, the preservation number ...
Embodiment 3
[0035] The difference from Example 2 is that
[0036] Take Trichoderma virens (Trichoderma virens) Y13-3 bacterial strain that grows well on the plate, cut into small pieces and inoculate in Jerusalem artichoke glucose liquid medium, put 300 ml of medium in each 1 liter Erlenmeyer flask, 100 bottles in total, room temperature Static fermentation for 40 days, filtration and collection of mycelia and fermentation broth respectively.
[0037] The Jerusalem artichoke glucose liquid medium is composed of 500 milliliters of boiled juice containing 100 grams of Jerusalem artichoke tubers per liter, 20 grams of glucose, 5 grams of peptone, 5 grams of yeast extract powder, and 500 milliliters of aged sea water.
[0038] Collect about 30 liters of fermented liquid, extract three times with ethyl acetate, and concentrate under reduced pressure; the mycelium is dried and pulverized, extracted three times with ethyl acetate, and concentrated under reduced pressure; the concentrate is detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com