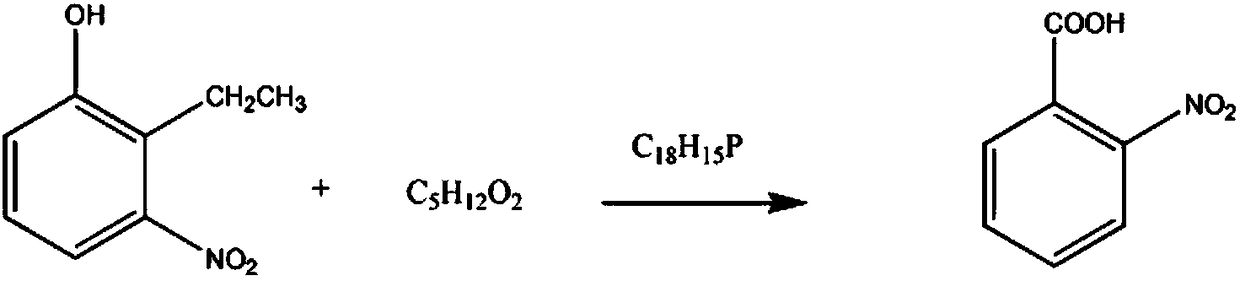Synthesis method of antagonist medicine intermediate o-nitrobenzoic acid
A technology for the synthesis of nitrobenzoic acid and its synthesis method, which is applied in the field of synthesis of o-nitrobenzoic acid, an intermediate of antagonist drugs, can solve the problems of complex process and low final yield, reduce intermediate links, shorten reaction time, The effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0010] Add 3mol of 2-ethyl-3-nitrophenol into the reaction vessel, 4mol mass fraction of 75% ethylene glycol monopropyl ether solution, raise the temperature of the solution to 30°C, control the stirring speed at 150rpm, keep it for 30min, and then Add 6 mol of triphenylphosphine powder, continue to react for 60 minutes, reduce the solution temperature to 5°C, precipitate solids, filter, wash with 85% propionic anhydride solution in mass fraction, and 91% triethylamine solution in mass fraction, and then Recrystallize in a 2-methylpentane solution with a mass fraction of 90%, and dehydrate with a dehydrating agent to obtain 445.89 g of crystalline o-nitrobenzoic acid, with a yield of 89%.
example 2
[0012] Add 3 mol of 2-ethyl-3-nitrophenol and 4.5 mol of ethylene glycol monopropyl ether solution with a mass fraction of 78% in the reaction vessel, raise the temperature of the solution to 32° C., control the stirring speed at 160 rpm, and keep it for 40 min. Then add 6.5 mol of triphenylphosphine powder, continue to react for 70min, lower the solution temperature to 6°C, precipitate solid, filter, wash with propionic anhydride solution with mass fraction of 88%, and triethylamine solution with mass fraction of 92% Wash, then recrystallize in 2-methylpentane solution with a mass fraction of 93%, and dehydrate with a dehydrating agent to obtain 460.92 g of crystalline o-nitrobenzoic acid, with a yield of 92%.
example 3
[0014] Add 3mol of 2-ethyl-3-nitrophenol into the reaction vessel, 5mol mass fraction of 80% ethylene glycol monopropyl ether solution, increase the solution temperature to 35°C, control the stirring speed at 170rpm, keep it for 50min, and then Add 7 mol of triphenylphosphine powder, continue the reaction for 90 minutes, lower the solution temperature to 8°C, precipitate solids, filter, and wash successively with 90% propionic anhydride solution and 94% triethylamine solution, Then recrystallize in 2-methylpentane solution with a mass fraction of 95%, and dehydrate with a dehydrating agent to obtain 475.95 g of crystalline o-nitrobenzoic acid with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com