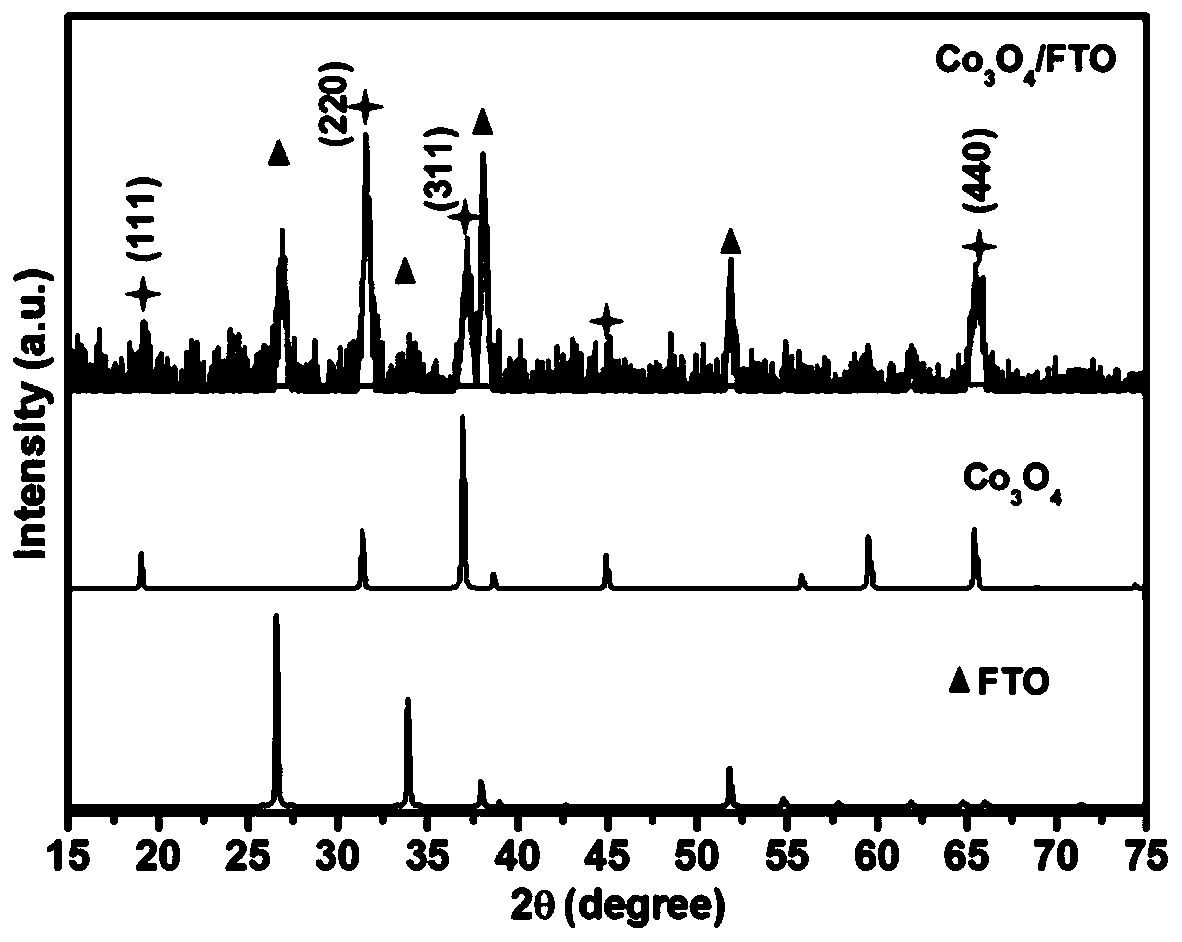
An efficient and stable co 3 o 4 Nanoribbon Array Chlorine Analysis Electrode
An array electrode and nanobelt technology, which is applied in the field of anode design for chlorine evolution reaction, can solve the problem that the anode is not involved, and achieve the effects of high commercial application prospect, simple preparation method and low overpotential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Co involved in the present invention 3 o 4 A method for preparing a nanoribbon array electrode, the steps are as follows:
[0043] (1) Add 80 ml of deionized water, 5 mmol of cobalt nitrate, 25 mmol of urea, 10 mmol of ammonium fluoride and 5 mg of polyethylene glycol into a 100 ml polytetrafluoroethylene-lined reactor. After stirring and dissolving, put the cleaned titanium mesh (or titanium sheet; copper sheet; copper mesh; carbon mesh; carbon cloth; carbon fiber; graphene; FTO glass and other substrates) into the reaction kettle. Seal the reaction kettle and place it in an oven at 120°C for 4 hours to react, take it out, and take out the titanium mesh (or titanium sheet; copper sheet; copper mesh; carbon mesh; carbon cloth; carbon fiber; graphene; FTO glass and other substrates after naturally cooling to room temperature) ), rinsed and dried with deionized water.
[0044] (2) Put the dried titanium mesh (or titanium sheet; copper sheet; copper mesh; carbon mesh; c...
Embodiment 1
[0050] 80 ml of deionized water, 5 mmol of cobalt nitrate, 25 mmol of urea, 10 mmol of ammonium fluoride and 5 mg of polyethylene glycol were added to a 100 ml polytetrafluoroethylene-lined reaction kettle. After stirring and dissolving, put the cleaned titanium mesh into the reaction kettle. Seal the reaction kettle and place it in an oven at 120°C for 4 hours to react, take it out, cool it down to room temperature naturally, take out the titanium mesh, wash and dry it with deionized water. The dried titanium mesh was put into a muffle furnace, and the temperature was raised to 350°C at a rate of 5°C / min, and kept for 2h. After the reaction, the titanium mesh was taken out and cooled naturally to obtain Co 3 o 4 Nanoribbon array electrodes. With Co 3 o 4 The nanoribbon array electrode is the working electrode, the saturated Ag / AgCl electrode is the reference electrode, and the Pt is the counter electrode. 100ml containing 20mmol sodium dihydrogen phosphate is the chlori...
Embodiment 2
[0057] 80 ml of deionized water, 5 mmol of cobalt nitrate, 25 mmol of urea, 10 mmol of ammonium fluoride and 5 mg of polyethylene glycol were added to a 100 ml polytetrafluoroethylene-lined reaction kettle. After stirring and dissolving, put the cleaned carbon cloth into the reaction kettle. Seal the reaction kettle and place it in an oven at 120°C for 4 hours to react, take it out, cool it down to room temperature naturally, take out the carbon cloth, wash and dry it with deionized water. The dried carbon cloth was placed in a muffle furnace, and the temperature was raised to 350 °C at a rate of 5 °C / min and kept for 2 h. After the reaction, the carbon cloth was taken out and cooled naturally to obtain Co 3 o 4 Nanoribbon array electrodes.
[0058] With Co 3 o 4 The nanoribbon array electrode is the working electrode, the saturated Ag / AgCl electrode is the reference electrode, and the Pt is the counter electrode. 100ml containing 20mmol sodium dihydrogen phosphate is th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com